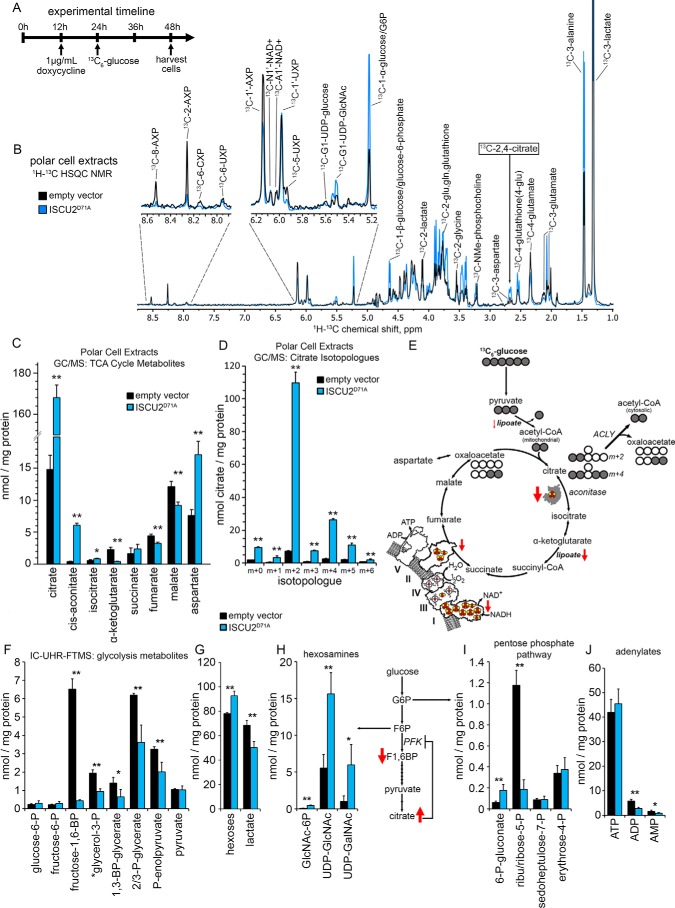
Figure 4.
Increased cellular citrate levels in Fe-S–deficient cells. A, HEK293 cells harboring either empty vector or ISCU2D71A were induced with doxycycline treatment 12 h before metabolic labeling for 24 h with 25 mm [13C6]glucose. B, incorporation of [13C6]glucose into cellular metabolites in cells expressing either empty vector or ISCU2D71A was assessed by 1H-13C HSQC NMR. Spectra are normalized to total cellular protein content measured by a BCA assay (see “Experimental procedures”). C, total cellular quantities of TCA cycle intermediates were assessed by GC-MS, revealing an 11.4-fold increase in intracellular citrate (n = 4, mean ± S.D. (error bars)). D, analysis of the fractional distribution of citrate isotopologues by GC-MS showed a 15.2-fold increase in the m+2 fraction of citrate derived from [13C6]glucose in cells expressing ISCU2D71A (n = 3, mean ± S.D.). E, the predominant metabolic pathway leading to increased citrate levels in cells expressing ISCU2D71A. The large increase in m+2 citrate suggested that the majority of the increased cellular citrate was a result of the combined action of pyruvate dehydrogenase and citrate synthase reactions together with the loss of aconitase activity. Shown is analysis of glycolytic intermediates by IC-UHR-FTMS (F); hexoses and lactate (G); hexosamines GlcNAc 1/6-phosphate (GlcNAc-P), UDP-GlcNAc, and UDP-GalNAc (H); and pentose phosphate pathway metabolites (I) (n = 4; percentage of control ± S.D.). J, levels of adenylates ATP, AMP, and ADP in empty vector control and ISCU2D71A cells. *, p < 0.05; **, p < 0.01.