FIG 1.
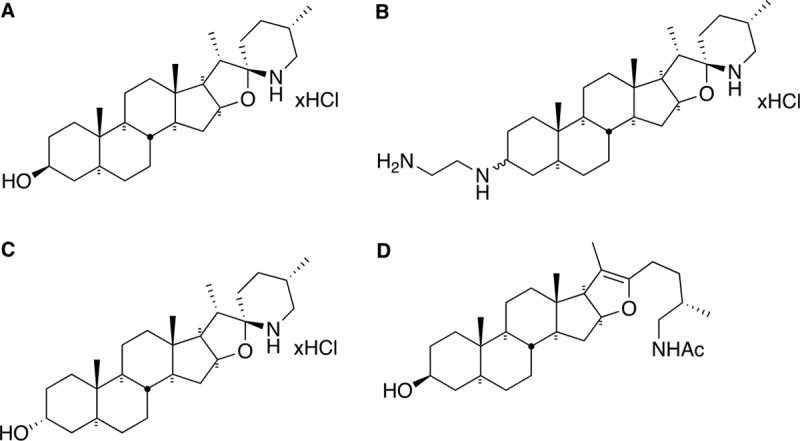
Structures of TO and analogs used in this study. (A) TO is characterized by 6 rings, 12 stereogenic centers, a 3 β-hydroxyl group, and spiro-fused rings in the form of an aminoketal. (B) FC04-100 contains a diamine in position 3. The two epimers in position 3 were separated as major (M) and minor (m) although due to the complexity of nuclear magnetic resonance signals, their respective structures could not be unambiguously assigned. (C) TO analog FC02-190 shows an α-hydroxyl group in position 3. (D) Analog FC04-116 shows an open spiroaminoketal moiety.
