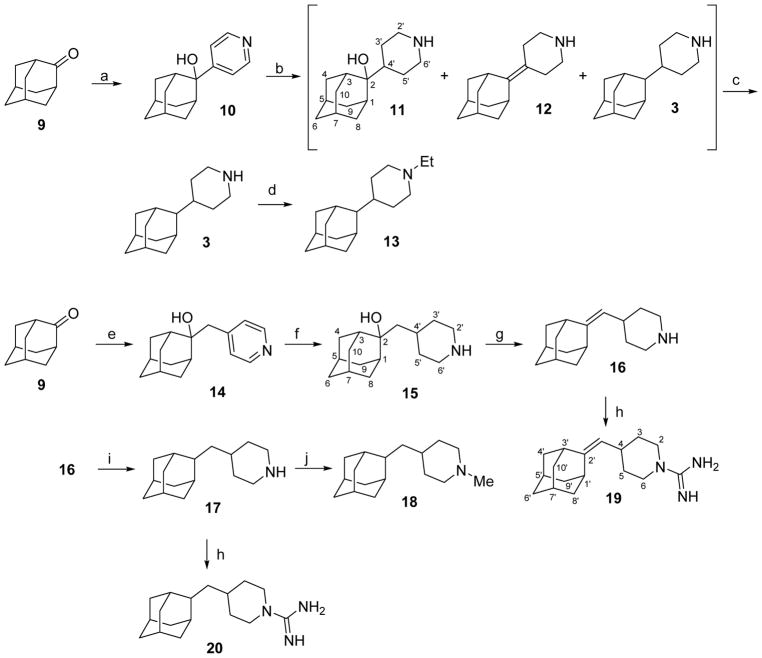
Scheme 2.
Synthesis of 4-(2-adamantyl)piperidines and related compounds from 2-adamantanone, 9.a
aReagents and conditions: a: 4-pyridyl lithium; Et2O/THF, −65 °C to rt, 70% yield; b: 1 atm H2, PtO2, ethanol, rt, 24 h, 93% yield of a mixture of 3, 11 and 12; c: 1) SOCl2, pyridine, anh. CH2Cl2, −60 °C, 30 min, 2) 1 atm H2, Pd/C, methanol, HCl, rt, 2 h, 63% overall yield; d: acetaldehyde, NaCNBH3, AcOH, methanol, rt, 24 h, 76% yield; e: 1) 4-picoline, anh THF, n-BuLi; 2) 9, 2 h, rt, 90% yield; f: 1 atm H2, PtO2, HCl, methanol, 5 days, > 99% yield; g: SOCl2, pyridine, anh. CH2Cl2, −60 °C, 30 min, > 99% yield; h: 1H-pyrazole-1-carboxamidine hydrochloride, anh Et3N, acetonitrile, 70 °C, 6 h, 88% yield for 19, 64% yield for 20; i: 1 atm H2, Pd/C, methanol, HCl, rt, 2 h, 68% yield; j: formaldehyde (37% aqueous solution), NaCNBH3, AcOH, rt, 18 h, 73% yield.