Fig. 3. Schema of mechanisms for the spread of pathological protein seeding in the brain via direct and indirect routes.
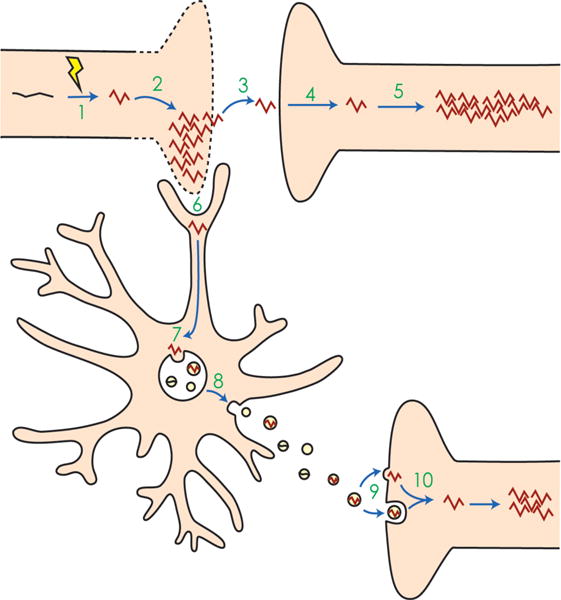
1 An event occurs that alters normal protein monomers, such as tau (black) into pathological misfolded proteins (red). In the case of tau, this event may be hyperphosphorylation. 2 The misfolded protein templates other monomers to misfold. Together, these misfolded proteins aggregate, eventually leading to cell death. 3 The misfolded protein escapes the dying cell and 4 is taken up by a nearby recipient cell. 5 In the recipient cell, the misfolded protein again templates other proteins to misfold and aggregate. 6 Alternatively, microglia phagocytose the cytopathic cells, thereby taking up the misfolded protein. 7 Misfolded proteins may be shuttled into endosomes and processed through endolysosomal degradation or incorporated into the multivesicular bodies (MVBs), where intraluminal vesicles package misfolded proteins and then 8 are released into the extracellular space as exosomes. 9 The exosomes may then be taken up by recipient cells via membrane fusion, directly releasing their cargo, or may be taken up by endocytosis. 10 The misfolded protein in the endocytosed exosome reaches the cytoplasm and templates and aggregates in the recipient cell. While in neuro-degenerative diseases such as Alzheimer’s and CTE this type of seeding is thought to occur across synapses, exosomes can also travel laterally or across long distances to be taken up by distal neurons or even other cell types, and cause pathological aggregation.
