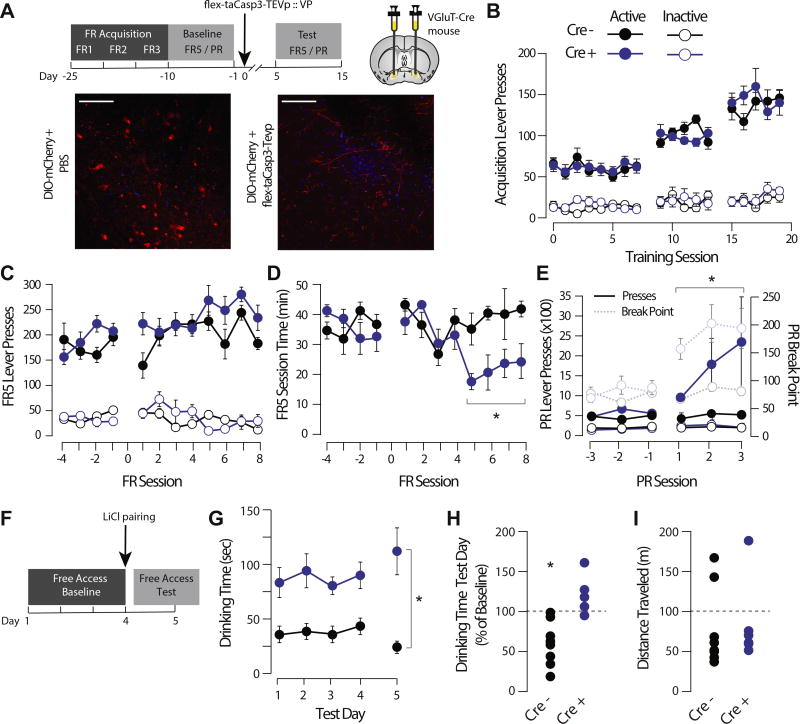
Figure 7.
Genetically encoded, caspase-mediated ablation of glutamatergic ventral pallidum (VP) neurons increases responding for sucrose and impairs sucrose taste aversion learning. (A) Experimental schematic. Mice were trained on an operant task to lever press for a sucrose pellet at fixed ratio (FR) 1 schedule. When performance stabilized on FR 1 responding, five sessions each of FR 2 and 3 schedules were completed before baseline operant testing (consisting of FR 5 and progressive ratio [PR] test sessions). VGluT2-Cre mice or Cre-negative control mice were then injected bilaterally with Cre-dependent viral caspase, and testing was resumed. Representative pictomicrographs showing floxed mCherry expression in the VP of unlesioned (left) and taCasp-lesioned (right) mice are shown. (B) There was no effect of genotype on the initial acquisition of the FR responding task (Fgenotype = 0.196, p = .662); lever presses increased as a function to FR schedule (Fsession = 314.117, p < .001), and there was a significant difference between active and inactive lever presses (Flever = 281.999, p < .001). (C) There was no effect of lesion on number of FR 5 lever presses (Cre− = pre 197.83 ± 9.5, post 264.13 ± 12.4 presses/session; Cre+ = pre 183.78 ± 10.65, post 214.53 ± 10.8 presses/session; Fsession × genotype = 2.612, p = .120). (D) Following lesion, the amount of time to earn 30 sucrose rewards decreased in VGluT2-Cre mice (Cre− = pre 36.09 ± 1.9, post 39.36 ± 2.54 min/session; Cre+ = pre 36.12 ± 2.6, post 22.04 ± 2.31 min/session; Fsession × genotype = 64.193, p = .003). (E) Following lesion, the number of lever presses in the PR task increased in VGluT2-Cre mice (Cre− = pre 372.11 ± 28.14, post 443.39 ± 32.9 presses/session; Cre+ = pre 496.24 ± 48.87, post 1656.62 ± 444.88 presses/session; Fsession × genotype = 5.198, p = .004), as did the breakpoint (Cre− = pre 14.47 ± 0.38, post 15.0 ± 0.41, Cre+ = pre 15.2 ± 0.3, post 19.0 ± 0.42, Fsession × genotype = 5.09, p = .001). (F) Schematic of sucrose aversion task. Lesioned mice were given free access to sucrose solution over a 4-day baseline. Sucrose solution was then paired with lithium chloride (LiCl), and mice were retested the following day. (G, H) Absolute time spent drinking sucrose was higher in VGluT2-Cre-lesioned mice (84.8 ± 8.4 s/session) than in wild-type control mice (40.3 ± 4.6 s/session). Wild-type mice decreased their sucrose consumption following LiCl pairing (50.3 ± 11.1% decrease from baseline; t7 paired t test = 2.844, p = .0249), whereas VGluT2-Cre-lesioned mice did not decrease their sucrose consumption in response to LiCl pairing (31.4 ± 9.4% nonsignificant change; t5 paired t test = 1.9024, p = .115). There was a significant effect of genotype and day by genotype by day interaction (Fgenotype = 39.034, p < .001; Fgenotype × test day = 9.603, p = .009. (I) There was no difference in spontaneous locomotor activity between lesioned WT (82.7 ± 20.8 m) and VGLuT2-Cre (77.7 ± 17.5 m) mice in an open field arena task (t12 = 0.184, p = .858). Scale bar = 50 µM.