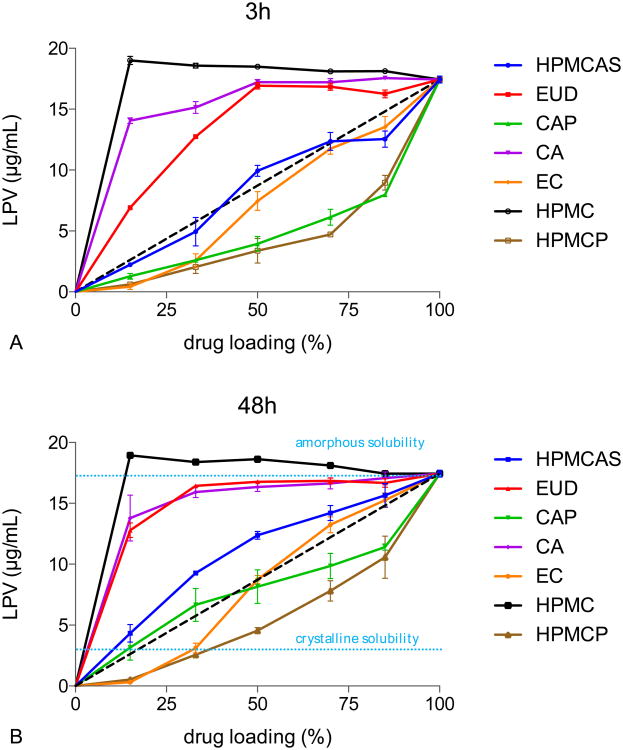
Figure 2.
LPV concentrations from LPV-polymer ASDs at pH 3 after A) 3h and B) 48h. dash line: ideal behavior. The amorphous solubility of lopinavir (17.4μg/mL) is used for the 100% drug loading point. 20 μg/mL of HPMC was added to the buffer to inhibit solution crystallization of lopinavir, thereby enabling the maximum release of LPV from the various amorphous solid dispersions to be directly compared.