Abstract
Objectives
Added dietary sugars contribute substantially to the diet of children and adolescents in the U.S., and recent evidence suggests that consuming sugar sweetened beverages (SSBs) during early life has deleterious effects on hippocampal-dependent memory function. Here we test whether the effects of early life sugar consumption on hippocampal function persist into adulthood when access to sugar is restricted to the juvenile/adolescent phase of development.
Methods
Male rats were given ad libitum access to an 11% weight-by-volume sugar solution (made with high fructose corn syrup-55) throughout the adolescent phase of development [post-natal day (PN) 26–56]. The control group received a second bottle of water instead, and both groups received ad libitum standard laboratory chow and water access throughout the study. At PN 56 sugar solutions were removed and at PN 175 rats were subjected to behavioral testing for hippocampal-dependent episodic contextual memory in the Novel Object in Context (NOIC) task, for anxiety-like behavior in the Zero Maze, and were given an intraperitoneal glucose tolerance test.
Results
Early life exposure to SSBs conferred long-lasting impairments in hippocampal-dependent memory function later in life- yet had no effect on body weight, anxiety-like behavior, or glucose tolerance. A second experiment demonstrated that NOIC performance was impaired at PN 175 even when SSB access was limited to 2 hours daily from PN 26–56.
Discussion
Our data suggest that even modest SSB consumption throughout early life may have long-term negative consequences on memory function during adulthood.
Keywords: sugar-sweetened beverages, SSB, obesity, hippocampus, programming, adolescence, fructose
Graphical abstract
Introduction
Consuming a “Western Diet”, high in saturated fat and added sugars, has deleterious effects on neurocognitive function (1–15), particularly for memory processes that rely on the integrity of the hippocampus (4, 16, 17). However, relatively little is known regarding how the individual components of a Western Diet impact cognitive heath. For example, a surfeit of added sugars (sugars added during food processing or preparation) contributes substantially to the modern food supply in the U.S. and in other Westernized countries. While several lines of research are actively investigating the metabolic consequences of excessive sugar consumption (for review see (18, 19)), the impact of added sugars (independent of elevated dietary fat and obesity) on cognitive function is less well understood.
In the U.S., children are the highest sugar consumers of any age group, with ~16% of their total daily caloric intake from added sugars (20, 21). This is particularly concerning given that the juvenile/adolescent period is a highly susceptible period for the onset of metabolic deficits resulting from excessive sugar intake (22), and new evidence suggests that this period of development is also a vulnerable time for sugar-related neurocognitive deficits (23, 24) (3). According to the 2005–2008 NHANES report, sugar-sweetened beverages (SSBs) are the source of over 40% of added sugar intake among youth (20). Given that the number of children aged 6–11 consuming SSBs has risen since the 1980s, and the kcal contribution of SSBs to the diet of juveniles rose by 60% between 1989 and 2008 (25), understanding the long-term impacts of SSB consumption on cognitive function later in life is increasingly critical.
Our lab and others have recently reported (using rodent models) that the juvenile/adolescent stage of development is a vulnerable period in which consuming dietary sugars negatively impacts the hippocampus (3, 23, 24), a brain region essential for learning and memory function that has more recently been linked with learned and rewarding aspects of feeding behavior (26). In rats, ad libitum access to an 11% high fructose corn syrup solution (HFCS-55) beginning during the juvenile period of development (immediately post weaning) but not beginning during early adulthood impairs hippocampal-dependent spatial memory in the Barnes Maze task and increases neuroinflammation in the hippocampus (3). It has also been reported that intermittent access to a 10% sucrose solution (2hr daily access) during the juvenile/adolescent period impairs hippocampal-dependent spatial learning and memory in the Morris Water Maze (23), as well as hippocampal-dependent object-in-place task (24). Relatively little is understood, however, as to whether these early life SSB-associated memory deficits can be reversed with dietary or other behavioral intervention. Therefore, using a rat model of juvenile/adolescent SSB consumption, we investigated whether impairments in hippocampal-dependent memory function due to early life SSB access persist well into adulthood, even after a long-term (~4.5 months) dietary intervention in which sugar is no longer available. In our previous report, juvenile/adolescent SSB access was associated hippocampal-dependent memory deficits, concomitant with impaired glucose tolerance when tested towards immediately following the juvenile/adolescent period (PN 60–70). Thus, in the present study we also administered an intraperitoneal glucose tolerance test (IP GTT) shortly after behavioral testing in adulthood (PN 175) to examine whether dietary intervention (SSB removal) can attenuate deficits in glucose metabolism associated with early life sugar consumption. Secondary outcome measures included testing for anxiety-like behavior (Zero Maze), energy intake, and body weight.
Methods
Experimental design
All experiments were performed in accordance with the approval of the Animal Care and Use Committee at the University of Southern California.
Cohort 1
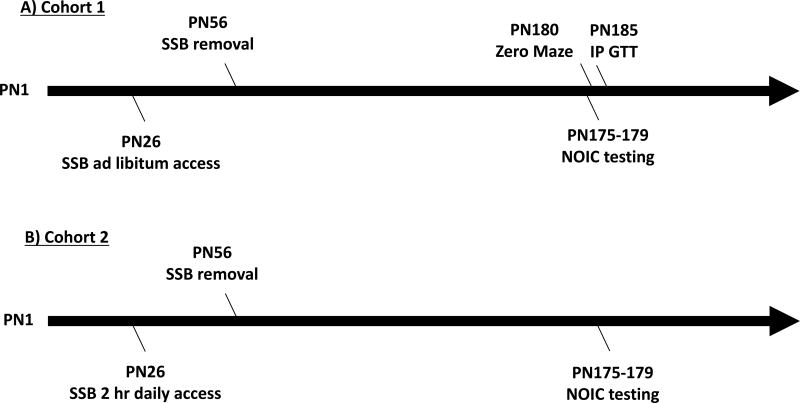
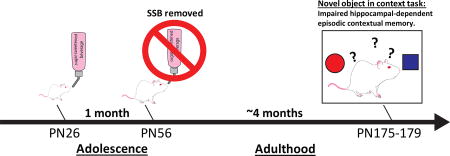
Twenty-four male Sprague Dawley rats (Envigo; post natal day (PN) 26; 50–70g) were housed individually in standard conditions with a 12:12 light/dark cycle and were divided into two groups based on their ad libitum access to: 1) 11% weight-by-volume (w/v) HFCS-55 solution diluted in reverse osmosis-filtered water (HFCS-ad lib; n=12) or 2) or an extra bottle of water (water; n=12). For the sugar group, the 11% w/v concentration of total sugar was chosen to be comparable to the amount of sugar in SSBs typically consumed by humans and also based on our prior data (3). In addition to the test solutions, all rats were given ad libitum access to Lab Diet 5001 (PMI Nutrition International, Brentwood, MO; 29.8 % kcal from protein, 13.4% kcal from fat, 56.7% kcal from carbohydrate) and water. Food intake, solution intake and body weights were monitored thrice weekly from PN 26–56, with additional recordings taken prior to behavioral testing. At PN 56 sugar solutions were removed and animals had access to water and chow only for the remainder of the study (Figure 1A). At PN 175, rats underwent Novel Object in Context (NOIC) testing, to measure hippocampal-dependent episodic contextual memory (described in detail below). Following NOIC testing, at PN 180, rodents underwent anxiety testing in the Zero Maze. At PN 185 an intraperitoneal glucose tolerance test (IP GTT) was performed in order measure glucose disposal as an estimated measure of insulin sensitivity.
Figure 1. Timeline of experiments.
A timeline of experiments for rats given either ad libitum (A) or 2-hour intermittent access (B) of 11 % w/v HFCS-55 solution (SSB) from postnatal day (PN) 26 to PN 56. Rats in (A) underwent Novel Object in Context (NOIC) testing from PN 175–179 and Zero Maze testing at PN 180. Intraperitoneal glucose tolerance test (IP GTT) was administered at PN 185. Rats in (B) underwent NOIC at PN 175–179.
Cohort 2
Twenty-two juvenile, male Sprague Dawley rats (Envigo; post natal day (PN) 26; 50–70g) were housed individually in standard conditions with a 12:12 light/dark cycle similar to Cohort 1. Rats were classified into two groups based on 2 hour daily exposure to solution feeding of: 1) 11% w/v HFCS 55 solution (HFCS 2 hr; n=11) or 2) water only (water; n=11). The control group was given a second water bottle. The second water bottle and the SSB were given to the rats four hours into the light cycle and removed 2 hours later. The timing of scheduled SSB feeding was chosen to be similar to prior publications where intermittent SSB access conferred hippocampal-dependent memory impairments (24). In addition to the test solutions, rats were given ad libitum access to Lab Diet 5001 (PMI Nutrition International, Brentwood, MO; 29.8 %kcal from protein, 13.4% kcal from fat, 56.7% kcal from carbohydrate) and water throughout the study. Food intake, solution intake, and body weights were monitored thrice weekly from PN 26–56, with additional recordings taken prior to behavioral testing. At PN 56 sugar solutions were removed and animals had access to water only for the remainder of the study (Figure 1B). At PN 175, rats underwent NOIC testing, to measure hippocampal-dependent episodic contextual memory, which is described in detail below. Zero maze testing and IP GTT were not examined in Cohort 2 based on the results from Cohort 1 (described below).
IP glucose tolerance test (IP GTT)
Animals were food restricted 24 hours prior to IP GTT. Immediately prior to the test, baseline blood glucose readings were obtained from tail tip and recorded by a blood glucose meter (One touch Ultra2, LifeScan Inc., Milpitas, CA). Each animal was then intraperitoneally (IP) injected with dextrose solution (0.923g/ml by body weight) and blood glucose readings were obtained at 30, 60, 90, and 120 min after IP injections, as previously described (3).
Zero Maze
To test for potential anxiety effects associated with SSB consumption, rats underwent testing in a Zero Maze. The Zero Maze is an elevated circular track (63.5 cm fall height, 116.8cm outside diameter), divided into four equal length sections. Two sections were open with 3 cm high curbs, whereas the 2 other closed sections contained 17.5 cm high walls. Animals were placed in the maze for 5 min while the experimenter records the total time spent in open sections (defined as the head and front two paws in open arms) as previously described (3).
Novel object in context task (NOIC)
NOIC measures episodic contextual memory by investigating the capacity to identify the novelty of a familiar object placed in a context in which it has not previously been paired. Procedures were adapted from prior reports (27, 28). Briefly, rats are habituated for 5-min sessions to two distinct contexts on subsequent days (with the habituation order counterbalanced by group): Context 1 is a semi-transparent box (15in W × 24in L × 12in H) with orange stripes and Context 2 is a grey opaque box (17in W × 17in L × 16in H) (Context identify assignments counterbalanced by group). Day 1 of NOIC begins with each animal being placed in Context 1 containing two distinct objects placed in opposite corners: a 500ml jar filled with blue water (Object A) and a square glass container (Object B) (Object identify assignments counterbalanced by group). On day 2 of NOIC, animals are placed in Context 2 with duplicates of Object A. On NOIC day 3, rats are placed in Context 2 with Objects A and Object B (which is not novel per se, but its placement is novel to Context 2). Sessions are 5 minutes long and are video recorded. The time spent investigating each object is recorded from the video recordings by an experimenter who is blinded to the treatment groups. Exploration is defined as sniffing or touching the object with the nose or forepaws. The task is scored by calculating the time spent exploring Object B divided by the time spent exploring both Objects A and B combined, which is the novelty or “discrimination index” [Exploration of Object B/ (Exploration of Object A + Object B)]. Rats with intact hippocampi will preferentially investigate Object B given that Object B is a familiar object yet is now presented in a novel context for that Object, whereas hippocampal inactivation impairs the preferential investigation of Object B in Context 2 (27).
Statistics
Data are presented as means ± SEM. For analytic comparisons of body weight, total food intake, and chow intake, groups were compared using Two-way mixed ANOVA with time as a within subjects factor and sugar as a between subjects factor. Data were analyzed in Prism software (GraphPad Inc., version 7.0). When significant differences were detected, Sidak post-hoc test for multiple comparisons was used. Area under the curve (AUC) for the IP GTT testing was also calculated using Prism. All other statistical analyses were performed using Student’s two-tailed unpaired t tests in excel software (Microsoft Inc., version 15.26). For all analyses, statistical significance was set at P<.05.
Results
Metabolic measures in juvenile/adolescent rats fed ad-libitum sugar solution
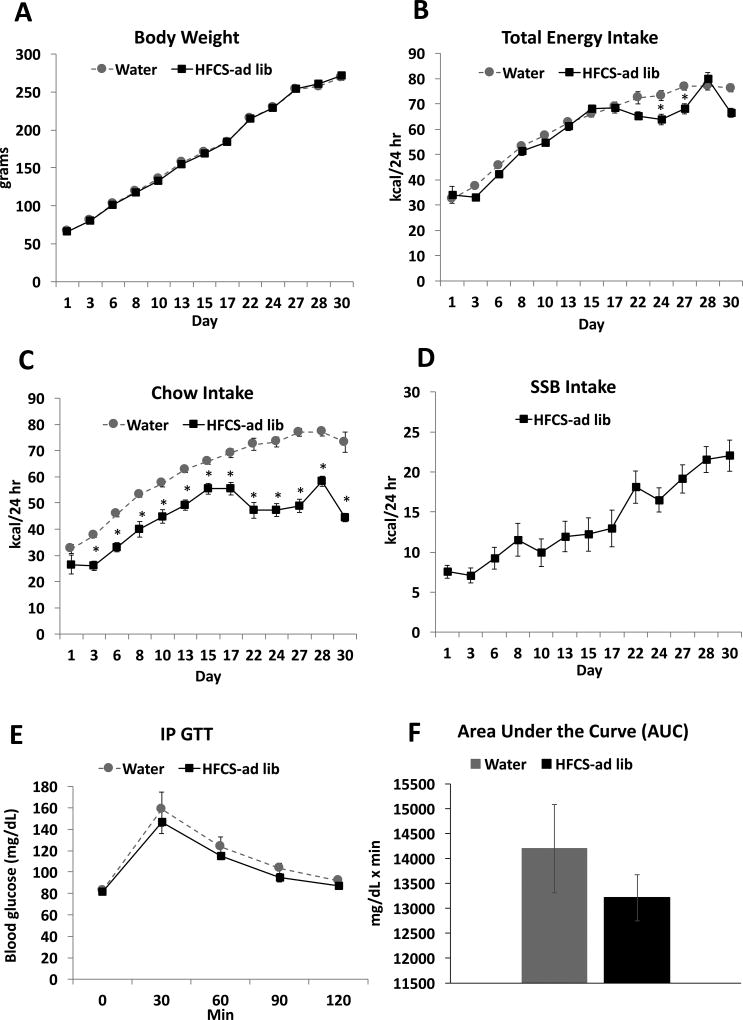
Ad libitum consumption of SSBs during the entire juvenile and adolescent phase of development did not result in significant differences in body weight during the access period (Figure 2A). There was a significant interaction (time × group) for total kcal intake (F(12, 264) = 3.75; P< .0001) with a main effects of time (F(12, 264) = 188.9; P< .0001) but not group (F(1, 22) = 3.49; P = n.s.) (Figure 2B). Post hoc-analyses revealed that animals consuming SSBs consumed fewer calories than the control group toward the end of the treatment period, on days 24 and 27. The lack of difference in caloric intake until the end of the treatment period was due to compensatory reductions in chow intake (Figure 2C), for which there was also a significant interaction (time × group; F(12, 264) = 10.51; P< .0001) with main effects of time (F(12, 264) = 113.60; P< .0001) and group (F(1, 22) = 73.14; P< .0001) (Figure 2C). Chow intake was significantly lower in the SSB treatment group than in the control group for the entire treatment period, with the exception of the first day of access to the treatment solutions. Intake of sugar solution steadily increased throughout the treatment period, with a mean kcal intake of 22.02 ± 1.94 on day 30 of access compared with 7.53 ± 0.80 on day 1 (Figure 2D; Student’s paired t-test; P< .0001). On average, animals given ad libitum access to the SSB consumed 24.36 ± 2.23% of their daily energy intake from SSBs. In the IP GTT, both groups showed a normal return to baseline in plasma glucose levels within 2 hours post injection, and comparisons of the AUC for the glucose tolerance test revealed that there were no significant differences in glucose tolerance resulting from SSB intake (Figure 2E and F).
Figure 2. Metabolic measures and dietary intakes in juvenile/adolescent rats fed ad-libitum sugar solution.
Body weights (A), total kcal intake (chow + sugar solutions; B), chow intake (C) and sugar intake (D) for animals who received ad libitum access to 11% w/v HFCS-55 (HFCS-ad lib) or a second water bottle for the control group (control). Results from an intraperitoneal glucose tolerance testing (IP GTT; D–E), showed no differences between the two treatment groups. Data are means ± SEM; n=12/group, *P< .05. SSB: sugar sweetened beverages.
Behavioral measures in adult rats exposed to SSBs ad libitum during the juvenile/adolescent phase of development
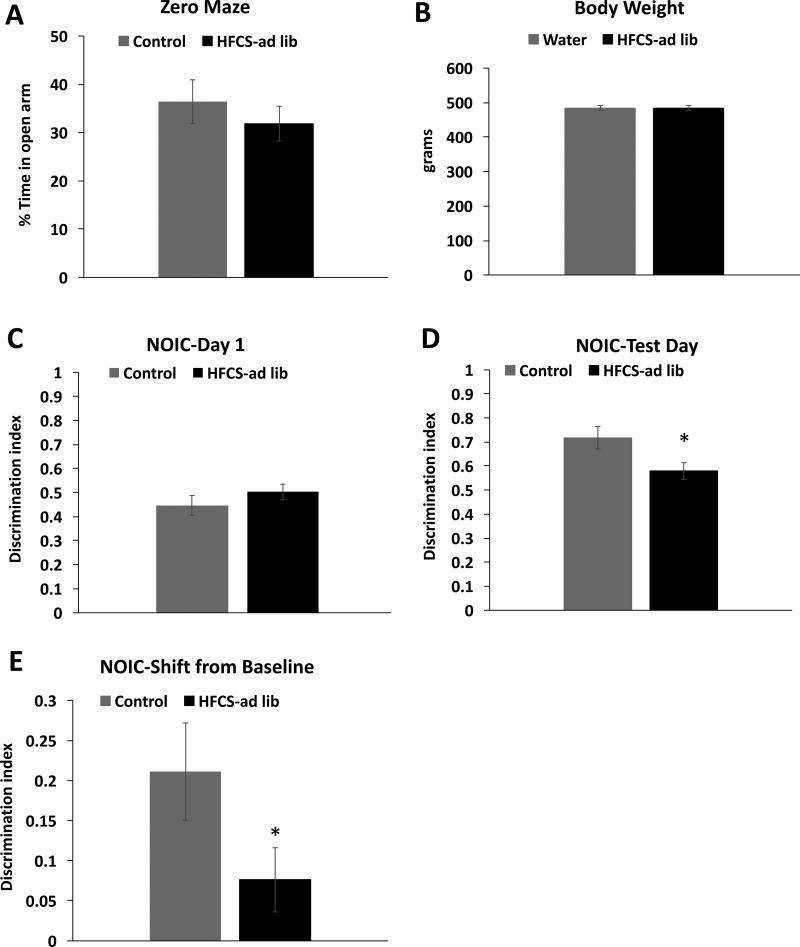
No significant group differences were observed in anxiety-like behavior in the Zero Maze test (Figure 3A). There were also no differences in body weight between the groups on PN 175 just prior to NOIC testing (Figure 3B). Results from the NOIC task revealed that, while there was no baseline preference for one object over the other on day 1 of the task (Figure 3C), rats exposed to SSBs during the juvenile/adolescent phase of development were significantly impaired in hippocampal-dependent episodic contextual memory at PN 179, as evidenced by a reduced discrimination index and a significantly lower shift from baseline discrimination index relative to controls (0.21 ± 0.06 (SSB group) vs 0.08 ± 0.04 (water group), P = .01; Figure 3D–E). It is important to note that there were no differences in total object exploration time (investigating either object) on day 1 of the task between the SSB group and controls, indicating that there were no group differences in novelty exploration (data not shown).
Figure 3. Behavioral measures in adult rats that were fed ad-libitum sugar solutions during the juvenile/adolescent phase of development.
Zero Maze test for anxiety (A) body weight at postnatal day 175 (B) Discrimination index results from Day 1 and test day of Novel Object in Context testing (NOIC) (C–D), and results from NOIC calculated as the shift from baseline discrimination index (E) in HFCS-ad lib rats. Data are means ± SEM; n=12/group, *P < .05.
Metabolic measures in juvenile/adolescent rats fed sugar solutions for 2 hours daily
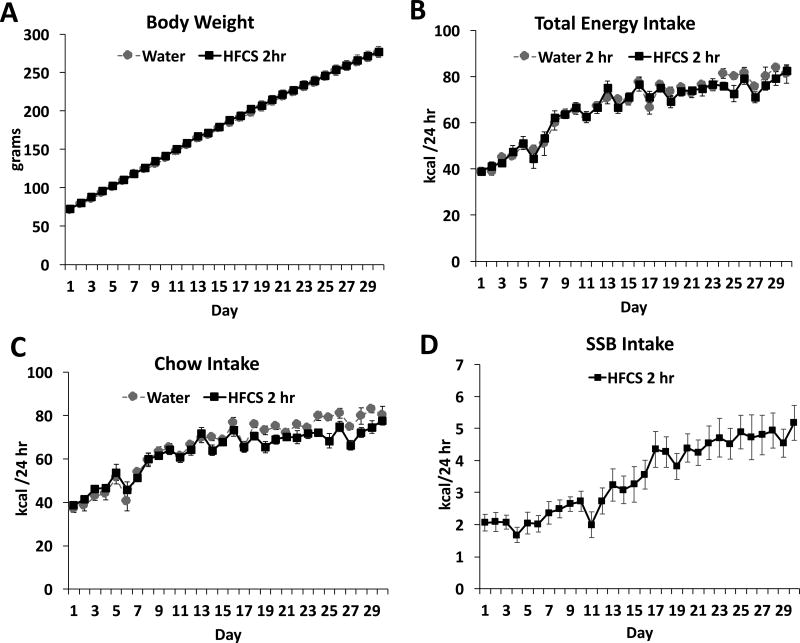
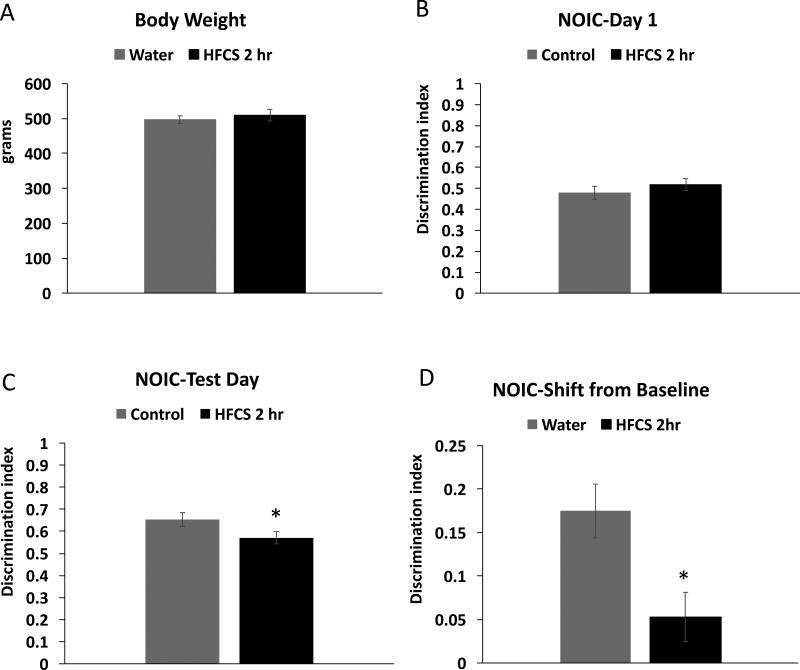
Similar to the ad libitum access group, consumption of SSBs for a restricted period of 2 hours/day during the entire juvenile/adolescent phase of development did not result in significant differences in body weight gain (Figure 4A), nor were there significant group differences in total kcals consumed (Figure 4B). There was a significant interaction for chow intake (time × group; F(29, 580) = 2.06; P= .001) with main effects of time (F(29, 580) = 71.85; P< .0001) but not group on kcal intake from chow (Figure 4C). Intake of sugar solution steadily increased throughout the treatment period, with a mean kcal intake of 5.18 ± 0.55 on day 30 of access compared with 2.06 ± 0.26 on day 1 (Figure 4D; Student’s paired t-test; P< .0001) Over the treatment period, rats given 2 hr of SSB access a day consumed an average of 5.03 ± 0.43 % of their kcal from the SSB.
Figure 4. Metabolic measures and dietary intakes in juvenile/adolescent rats fed sugar solution with intermittent 2-hour daily access.
Body weights (A), total kcal intake (chow + sugar solutions; B), chow intake (C) and sugar intake (D) for animals who received intermittent, 2-hour daily access to 11% w/v high fructose corn syrup solution (HFCS 2 hr) or a second water bottle for the control group (control). Data are means ± SEM; n=11/group, *P< .05 SSB: sugar sweetened beverages.
NOIC behavioral testing in adult rats exposed to SSBs for 2 hours daily during the juvenile/adolescent phase of development
There were no differences in body weight between the groups at the time of NOIC testing, which was from PN 175–179 after ~4.5 months without access to SSBs (Figure 5A). Results from the NOIC task revealed that while there was no baseline preference for one object over the other on day 1 of the task (Figure 5B), rats exposed to SSBs for 2 hours daily during the adolescent phase of development were significantly impaired in hippocampal-dependent episodic contextual memory at PN 179, as evidenced by a reduced discrimination index and a significantly lower shift from baseline discrimination index relative to controls (0.17 ± 0.03 (SSB group) vs 0.05 ± 0.03 (water group), P = .009; Figure 5C–D).
Figure 5. Novel Object in Context (NOIC) in adult rats that were given intermittent sugar solution access for 2-hours daily during the juvenile/adolescent phase of development.
Body weight at postnatal day 175 (A) Discrimination index results from Day 1 and test day of Novel Object in Context testing (NOIC) (B–C), and results from NOIC calculated as the shift from baseline discrimination index (D) in rats given 2-hour daily access to a solution of 11% w/v high fructose corn syrup solution (HFCS 2 hr) or a second water bottle for the control group (control). Data are means ± SEM; n=12/group, *P< .05.
Discussion
Results from the rodent experiments presented herein show that consumption SSBs during the juvenile/adolescent phase of development significantly impairs hippocampal-dependent episodic contextual memory when tested much later in life following an extended period (~4.5 months) without SSB access. These memory deficits persisted even when SSB access was intermittent/restricted to 2 hours per day, resulting in ~5% of total kcal from sugar consumption. Furthermore, memory impairments were observed independent of metabolic abnormalities such as body weight gain and glucose intolerance. These findings reveal that the juvenile/adolescent period is one of high vulnerability to long-lasting cognitive impairments resulting from habitual sugar consumption.
In our previous report (3), 11% HFCS-55 access during the juvenile/adolescent period significantly impaired hippocampal-dependent spatial memory when tested from PN 60–70, while having no effect on anxiety-like behavior in the Zero Maze. Similar to this previous study, HFCS-55 had no effect on body weight during the access period (nor at the time of NOIC testing), nor was there an effect on Zero Maze performance in the present study. The rationale for assessing Zero Maze performance behavior is that the hippocampus, particularly the ventral subregion, is linked with anxiety-like behavior in rodents (29), and potential group differences in anxiety can complicate interpretation of the NOIC data. The lack of group differences in Zero Maze performance in the present study combined with group differences in NOIC (a dorsal hippocampal-dependent memory task (28) suggests the dorsal subregion may be more vulnerable than the ventral subregion to disruption by early life SSB consumption. However, more research is required to explore this possibility. Moreover, there is little consensus in the literature regarding the precise differentiation of function between the dorsal and ventral hippocampal subregions (29–31).
One key difference between the present results and our previous report is that in our previous report, the HFCS-55-exposed rats demonstrated impairments in IP GTT relative to controls when tested at ~PN 70 whereas in our current report the ad libitum SSB group in the present study were impaired in hippocampal-dependent memory without concomitant IP GTT deficits at PN 185 (following an extended period without SSB access). Thus, dietary intervention (removal of SSB for ~4.5 months) appears to be effective in reversing impaired glucose tolerance (at least when measured with IP GTT), but is not effective in reversing hippocampal-dependent memory deficits. Collectively these findings suggest that the hippocampal dysfunction associated with early life SSB consumption in rats is not easily reversible with dietary intervention, and is unlikely to be related to SSB effects on peripheral glucose metabolism.
The juvenile/adolescent period of development is one of rapid growth and maturation and is a particularly critical period for hippocampal development (32–36). The hippocampus is highly susceptible to the deleterious effects of obesogenic dietary factors (2, 6–8), as several recent studies have revealed that juvenile and adolescent obesogenic dietary factors such as palatable high fat/high sugar diets (HFD) negatively impact hippocampal function. For example, juvenile/adolescent mice fed a HFD (45% kcal from fat) beginning at 5 weeks old showed impairments in the hippocampal dependent Novel Location Recognition task, whereas mice that were given the diet at 8 weeks old were not impaired (37). Similarly, in juvenile but not adult rats, consumption of a HFD (45% kcal from fat) significantly impaired hippocampal-dependent spatial memory retention and spatial reversal learning (38), and mice fed a HFD for 11 weeks post weaning (a period spanning the entire juvenile adolescent period and ~7 weeks into young adulthood) were impaired in hippocampal dependent relational memory flexibility assessed in a Two-stage Radial Arm Maze Concurrent Spatial Discrimination task, whereas adults exposed to the diet for a similar amount of time were not impaired (39). The reproducibility of these outcomes using several different hippocampal-dependent memory tasks strongly suggests the capacity for dietary and metabolic factors to negatively impact hippocampal function when exposure to these factors occurs during early life. A recent study showed that early life exposure to a HFD in mice in sufficient quantities as to induce diet-induced obesity and insulin resistance during the juvenile/adolescent phase of development had long-lasting negative implications for hippocampal function that were not reversible by switching animals to a low-fat diet during adulthood. Interestingly, however, the dietary intervention reversed the effects of the HFD on body weight and glucose metabolism (40). Our data isolate one component of the HFD (added sugars) that is sufficient on its own to promote similar deleterious effects on hippocampal function, even following extended dietary intervention. We also note that the prefrontal cortex (PFC) is another brain region that appears to be vulnerable to obesogenic dietary factors when consumed during early life periods. For example, Reichelt and colleagues observed deficits in a PFC-dependent rodent Stroop task in rats given intermittent (2 hours daily) access to sucrose during adolescence (24). In addition, Labouesse, Meyer, and colleagues demonstrated that mice given a high-fat diet during adolescence were impaired in a PFC-dependent cognitive flexibility task, whereas no impairments were observed when the high-fat diet was given during adulthood at PN 70 (41).
While sugar is often a component of the palatable obesogenic HFDs used for research purposes, until recently few studies investigated the effects of dietary sugar per se on hippocampal function. Jurdak and Kanarek reported that free access to a 32% sucrose solution in adult rats produced body weight gain and impaired novel object recognition relative to controls (42). Hsu et al., reported that juvenile and adolescent, but not adult, ad libitum consumption of an 11% high fructose corn syrup (HFCS-55) solution for 30 days impaired hippocampal-dependent spatial learning and memory retention in rats (3). However, no effect of juvenile/adolescent HFCS-55 access was observed in a hippocampal independent response learning task of comparable difficulty that was performed under similar conditions and using similar motivational tactics but did not require the utilization of spatial cues (3). Using a separate spatial learning and memory task (the Morris Water Maze) and an intermittent access (2hrs daily) model of 10% sucrose solution for 28 days, Kendig et al. observed that both juvenile/adolescent and young adult exposure to the intermittent sucrose impaired hippocampus dependent spatial learning and memory (23). Our current finding that early life access to 11% HFCS solution for just 2 hours/day impairs hippocampal-dependent episodic contextual memory is in agreement with this study, as well as another recent study from Reichelt et al., where rats were exposed to 10% sucrose for 2 hours a day during the juvenile and adolescent period and showed an impaired memory capacity in an object-in-place task, which tests hippocampal-dependent episodic contextual memory (24). In this study the sucrose treatment stopped at PN 56 and rats were tested at PN 92, 5-weeks after the last sucrose treatment when the animals are in early adulthood (24). Our results extend these findings and show, remarkably, that impairments persist for 4.5 months after removing SSB access at the end of the adolescent period, suggesting that the damage imparted by early life HFCS SSB exposure is not reversible by withdrawing sugar access alone. One limitation of our current findings is the use of only one hippocampal-dependent memory task. However, the notion that early life sugar consumption produces hippocampal deficits is further supported by the aforementioned studies (23, 24) that utilized different hippocampal-dependent tasks, as well as our previous findings that early-life sugar consumption impairs hippocampal-dependent spatial memory in the Barnes Maze task (3). Future research will be needed to determine whether early life sugar consumption confers long-lasting impairments in hippocampal-dependent spatial memory.
While obesity and elevated BMI are associated with cognitive deficits (43, 44), our data suggest that weight gain is not necessary for sugar induced cognitive deficits caused by early life sugar consumption. Similarly, while obesity per se is associated with reduced hippocampal volume (45) and impaired hippocampal function (46–48), evidence shows that dietary factors can negatively impact hippocampal function independent of obesity. For example, hippocampal-dependent spatial memory impairments have been reported in rats following Western Diet consumption even before the onset of diet-induced obesity or weight gain (49) (50). Similar to Western diets, high fructose diets can also impair hippocampal-dependent learning and memory in rodents independent of obesity (3, 7, 51–54). Our data corroborate and extend the work of others (3, 13) in showing that neither weight gain nor peripheral glucose intolerance is a necessary component of sugar-induced cognitive deficits.
The neurobiological mechanisms underlying long-lasting hippocampal-dependent memory deficits resulting from early life sugar consumption are poorly understood. One possibility is that the long-term dietary sugar “programming” of behavioral deficits involves changes in the brain epigenome. Indeed, a recent study reported in mice that high-fat diet-induced obesity altered DNA methylation in memory-associated genes in the hippocampus, including Sirtuin 1 and reelin, and these effects were functionally relevant to high-fat diet-related memory deficits in a hippocampal-dependent object location memory task (55). Another possibility is that early life habitual sugar consumption produces long-lasting changes in the gut microbiome (the collective genome of microbes residing in the gastrointestinal tract) that contribute to negative cognitive outcomes. Several recent findings are consistent with this possibility. For example, depleted gut microbiota during early adolescence in mice impairs novel object recognition and hippocampal-dependent social transmission of food preference, and colonization with commensal microbiota from a replete host normalizes these cognitive abnormalities (56). In addition, microbiome transplantation in mice consuming a healthy chow from donor mice fed a high-fat diet recapitulated high-fat diet-associated deficits in exploratory and stereotypical behaviors (57). Finally, we recently reported that ad libitum access to an 11% w/v sugar solution during the juvenile and adolescent period (modeling the approach in Cohort 1 in the present paper) significantly altered gut bacteria relative to controls at every phylogenetic level, with significant group effects in ~33% of gut bacteria at the family level (58). Follow-up research is warranted to investigate whether long-lasting memory deficits resulting from early life consumption of obesobenic dietary factors are related to changes in the brain epigenome and/or gut microbiome.
Conclusions
Results from this study suggest that consumption of an 11% HFCS solution during early life (childhood and adolescence) may confer long-lasting impairments in memory function independent of changes in body weight or aberrant glucose metabolism. Specifically, we found that early life consumption of SSBs in rats impaired contextual episodic memory, a mnemonic processes that relies on the integrity of the hippocampus (27). Strikingly, the impairments lasted well into late adulthood, even after SSB access was completely removed from the diet, and even when SSB access during the juvenile/adolescent period was restricted to 2 hours per day. These findings suggest that the mechanisms altering hippocampal function are not easily reversible by simply removing sugars from the diet. These results further suggest that young people may be at risk for long-term negative cognitive consequences from consuming SSBs, however, as we utilized a rodent model of SSB consumption during early life, further studies are necessary to infer whether these results are translatable to humans.
Acknowledgments
We would like to thank Andrea Suarez, Clarissa Liu, Alyssa Cortella, and Lilly Taing for their tremendous assistance with data collection.
Funding
Our funding sources are: NIDDK DK104897 (SK), University of Southern California Diabetes and Obesity Research Institute (SK), and NIDDK F32-DK111158 (EN).
Footnotes
Disclosures
We have no conflicts of interest to disclose.
References
- 1.Francis H, Stevenson R. The longer-term impacts of Western diet on human cognition and the brain. Appetite. 2013;63:119–28. doi: 10.1016/j.appet.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Davidson TL, Monnot A, Neal AU, Martin AA, Horton JJ, Zheng W. The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood-brain barrier integrity differ for diet-induced obese and diet-resistant rats. Physiology & behavior. 2012;107(1):26–33. doi: 10.1016/j.physbeh.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu TM, Konanur VR, Taing L, Usui R, Kayser BD, Goran MI, et al. Effects of sucrose and high fructose corn syrup consumption on spatial memory function and hippocampal neuroinflammation in adolescent rats. Hippocampus. 2015;25(2):227–39. doi: 10.1002/hipo.22368. [DOI] [PubMed] [Google Scholar]
- 4.Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiology & behavior. 2011;103(1):59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanoski SE, Meisel RL, Mullins AJ, Davidson TL. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behavioural brain research. 2007;182(1):57–66. doi: 10.1016/j.bbr.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J Alzheimers Dis. 2010;21(1):207–19. doi: 10.3233/JAD-2010-091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noble EE, Kanoski SE. Early life exposure to obesogenic diets and learning and memory dysfunction. Curr Opin Behav Sci. 2016;9:7–14. doi: 10.1016/j.cobeha.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noble EE, Mavanji V, Little MR, Billington CJ, Kotz CM, Wang C. Exercise reduces diet-induced cognitive decline and increases hippocampal brain-derived neurotrophic factor in CA3 neurons. Neurobiology of learning and memory. 2014;114C:40–50. doi: 10.1016/j.nlm.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112(4):803–14. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 10.Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gomez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123(2):429–40. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Beilharz JE, Kaakoush NO, Maniam J, Morris MJ. The effect of short-term exposure to energy-matched diets enriched in fat or sugar on memory, gut microbiota and markers of brain inflammation and plasticity. Brain, behavior, and immunity. 2016;57:304–13. doi: 10.1016/j.bbi.2016.07.151. [DOI] [PubMed] [Google Scholar]
- 12.Beilharz JE, Maniam J, Morris MJ. Short exposure to a diet rich in both fat and sugar or sugar alone impairs place, but not object recognition memory in rats. Brain, behavior, and immunity. 2014;37:134–41. doi: 10.1016/j.bbi.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Beilharz JE, Maniam J, Morris MJ. Short-term exposure to a diet high in fat and sugar, or liquid sugar, selectively impairs hippocampal-dependent memory, with differential impacts on inflammation. Behavioural brain research. 2016;306:1–7. doi: 10.1016/j.bbr.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Baym CL, Khan NA, Monti JM, Raine LB, Drollette ES, Moore RD, et al. Dietary lipids are differentially associated with hippocampal-dependent relational memory in prepubescent children. The American journal of clinical nutrition. 2014;99(5):1026–32. doi: 10.3945/ajcn.113.079624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan NA, Raine LB, Drollette ES, Scudder MR, Hillman CH. The relation of saturated fats and dietary cholesterol to childhood cognitive flexibility. Appetite. 2015;93:51–6. doi: 10.1016/j.appet.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu TM, Kanoski SE. Blood-brain barrier disruption: mechanistic links between Western diet consumption and dementia. Front Aging Neurosci. 2014;6:88. doi: 10.3389/fnagi.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin AA, Davidson TL. Human cognitive function and the obesogenic environment. Physiology & behavior. 2014;136:185–93. doi: 10.1016/j.physbeh.2014.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanhope KL. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit Rev Clin Lab Sci. 2016;53(1):52–67. doi: 10.3109/10408363.2015.1084990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reichelt AC. Adolescent Maturational Transitions in the Prefrontal Cortex and Dopamine Signaling as a Risk Factor for the Development of Obesity and High Fat/High Sugar Diet Induced Cognitive Deficits. Frontiers in behavioral neuroscience. 2016;10:189. doi: 10.3389/fnbeh.2016.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ervin RB, Kit BK, Carroll MD, Ogden CL. Consumption of added sugar among U.S. children and adolescents, 2005–2008. NCHS Data Brief. 2012;(87):1–8. [PubMed] [Google Scholar]
- 21.Ervin RB, Ogden CL. Consumption of added sugars among U.S. adults, 2005–2010. NCHS data brief. 2013;(122):1–8. [PubMed] [Google Scholar]
- 22.Goran MI, Dumke K, Bouret SG, Kayser B, Walker RW, Blumberg B. The obesogenic effect of high fructose exposure during early development. Nature reviews Endocrinology. 2013;9(8):494–500. doi: 10.1038/nrendo.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kendig MD, Boakes RA, Rooney KB, Corbit LH. Chronic restricted access to 10% sucrose solution in adolescent and young adult rats impairs spatial memory and alters sensitivity to outcome devaluation. Physiology & behavior. 2013;120:164–72. doi: 10.1016/j.physbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Reichelt AC, Killcross S, Hambly LD, Morris MJ, Westbrook RF. Impact of adolescent sucrose access on cognitive control, recognition memory, and parvalbumin immunoreactivity. Learning & memory. 2015;22(4):215–24. doi: 10.1101/lm.038000.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lasater G, Piernas C, Popkin BM. Beverage patterns and trends among school-aged children in the US, 1989–2008. Nutr J. 2011;10:103. doi: 10.1186/1475-2891-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanoski SE, Grill HJ. Hippocampus Contributions to Food Intake Control: Mnemonic, Neuroanatomical, and Endocrine Mechanisms. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez MC, Villar ME, Ballarini F, Viola H. Retroactive interference of object-in-context long-term memory: role of dorsal hippocampus and medial prefrontal cortex. Hippocampus. 2014;24(12):1482–92. doi: 10.1002/hipo.22328. [DOI] [PubMed] [Google Scholar]
- 28.Balderas I, Rodriguez-Ortiz CJ, Salgado-Tonda P, Chavez-Hurtado J, McGaugh JL, Bermudez-Rattoni F. The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learning & memory. 2008;15(9):618–24. doi: 10.1101/lm.1028008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nature reviews Neuroscience. 2014;15(10):655–69. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- 30.Jarrard LE, Luu LP, Davidson TL. A study of hippocampal structure-function relations along the septo-temporal axis. Hippocampus. 2012;22(4):680–92. doi: 10.1002/hipo.20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and biobehavioral reviews. 2000;24(4):417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 33.Sherman LE, Rudie JD, Pfeifer JH, Masten CL, McNealy K, Dapretto M. Development of the default mode and central executive networks across early adolescence: a longitudinal study. Dev Cogn Neurosci. 2014;10:148–59. doi: 10.1016/j.dcn.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature neuroscience. 1999;2(10):861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 36.Higuera-Matas A, Miguens M, Coria SM, Assis MA, Borcel E, del Olmo N, et al. Sex-specific disturbances of the glutamate/GABA balance in the hippocampus of adult rats subjected to adolescent cannabinoid exposure. Neuropharmacology. 2012;62(5–6):1975–84. doi: 10.1016/j.neuropharm.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 37.Valladolid-Acebes I, Fole A, Martin M, Morales L, Cano MV, Ruiz-Gayo M, et al. Spatial memory impairment and changes in hippocampal morphology are triggered by high-fat diets in adolescent mice. Is there a role of leptin? Neurobiology of learning and memory. 2013;106:18–25. doi: 10.1016/j.nlm.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Boitard C, Cavaroc A, Sauvant J, Aubert A, Castanon N, Laye S, et al. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain, behavior, and immunity. 2014;40:9–17. doi: 10.1016/j.bbi.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Boitard C, Etchamendy N, Sauvant J, Aubert A, Tronel S, Marighetto A, et al. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus. 2012;22(11):2095–100. doi: 10.1002/hipo.22032. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Freire D, Knable L, Zhao W, Gong B, Mazzola P, et al. Childhood and adolescent obesity and long-term cognitive consequences during aging. J Comp Neurol. 2015;523(5):757–68. doi: 10.1002/cne.23708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Labouesse MA, Lassalle O, Richetto J, Iafrati J, Weber-Stadlbauer U, Notter T, et al. Hypervulnerability of the adolescent prefrontal cortex to nutritional stress via reelin deficiency. Molecular psychiatry. 2017;22(7):961–71. doi: 10.1038/mp.2016.193. [DOI] [PubMed] [Google Scholar]
- 42.Jurdak N, Kanarek RB. Sucrose-induced obesity impairs novel object recognition learning in young rats. Physiology & behavior. 2009;96(1):1–5. doi: 10.1016/j.physbeh.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Dai Q, Jackson JC, Zhang J. Overweight is associated with decreased cognitive functioning among school-age children and adolescents. Obesity. 2008;16(8):1809–15. doi: 10.1038/oby.2008.296. [DOI] [PubMed] [Google Scholar]
- 44.Sellbom KS, Gunstad J. Cognitive function and decline in obesity. J Alzheimers Dis. 2012;30(Suppl 2):S89–95. doi: 10.3233/JAD-2011-111073. [DOI] [PubMed] [Google Scholar]
- 45.Jagust W, Harvey D, Mungas D, Haan M. Central obesity and the aging brain. Archives of neurology. 2005;62(10):1545–8. doi: 10.1001/archneur.62.10.1545. [DOI] [PubMed] [Google Scholar]
- 46.Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113(3):607–15. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 47.Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, et al. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behav Neurosci. 2005;119(5):1389–95. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- 48.Khan NA, Baym CL, Monti JM, Raine LB, Drollette ES, Scudder MR, et al. Central adiposity is negatively associated with hippocampal-dependent relational memory among overweight and obese children. The Journal of pediatrics. 2015;166(2):302–8. e1. doi: 10.1016/j.jpeds.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanoski SE, Davidson TL. Different patterns of memory impairments accompany short- and longer-term maintenance on a high-energy diet. Journal of experimental psychology Animal behavior processes. 2010;36(2):313–9. doi: 10.1037/a0017228. [DOI] [PubMed] [Google Scholar]
- 50.Murray AJ, Knight NS, Cochlin LE, McAleese S, Deacon RM, Rawlins JN, et al. Deterioration of physical performance and cognitive function in rats with short-term high-fat feeding. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23(12):4353–60. doi: 10.1096/fj.09-139691. [DOI] [PubMed] [Google Scholar]
- 51.Agrawal R, Noble E, Vergnes L, Ying Z, Reue K, Gomez-Pinilla F. Dietary fructose aggravates the pathobiology of traumatic brain injury by influencing energy homeostasis and plasticity. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016;36(5):941–53. doi: 10.1177/0271678X15606719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meng Q, Ying Z, Noble E, Zhao Y, Agrawal R, Mikhail A, et al. Systems Nutrigenomics Reveals Brain Gene Networks Linking Metabolic and Brain Disorders. EBioMedicine. 2016;7:157–66. doi: 10.1016/j.ebiom.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ross AP, Bartness TJ, Mielke JG, Parent MB. A high fructose diet impairs spatial memory in male rats. Neurobiology of learning and memory. 2009;92(3):410–6. doi: 10.1016/j.nlm.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ross AP, Bruggeman EC, Kasumu AW, Mielke JG, Parent MB. Non-alcoholic fatty liver disease impairs hippocampal-dependent memory in male rats. Physiology & behavior. 2012;106(2):133–41. doi: 10.1016/j.physbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 55.Heyward FD, Gilliam D, Coleman MA, Gavin CF, Wang J, Kaas G, et al. Obesity Weighs down Memory through a Mechanism Involving the Neuroepigenetic Dysregulation of Sirt1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36(4):1324–35. doi: 10.1523/JNEUROSCI.1934-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Desbonnet L, Clarke G, Traplin A, O'Sullivan O, Crispie F, Moloney RD, et al. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain, behavior, and immunity. 2015;48:165–73. doi: 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard Et, Taylor CM, Welsh DA, et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biological psychiatry. 2015;77(7):607–15. doi: 10.1016/j.biopsych.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noble EE, Hsu TM, Jones RB, Fodor AA, Goran MI, Kanoski SE. Early-Life Sugar Consumption Affects the Rat Microbiome Independently of Obesity. The Journal of nutrition. 2017;147(1):20–8. doi: 10.3945/jn.116.238816. [DOI] [PMC free article] [PubMed] [Google Scholar]