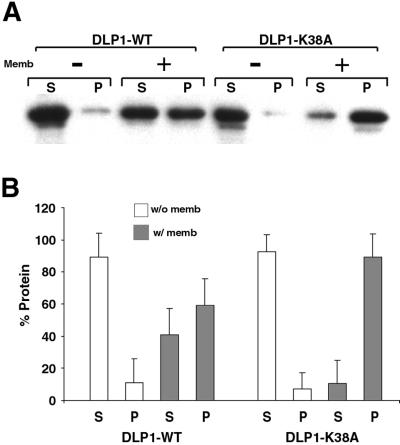
Figure 5.
DLP1-K38A has a high affinity for membranes. (A) Purified GST-tagged proteins were incubated with or without carbonate-stripped total rat liver membranes in the presence of GTP. Reaction mixtures were centrifuged at 39,000 × g to separate supernatant and pellet, which were then subjected to immunoblot analyses with an anti-DLP1 antibody. In the absence of membranes, WT and DLP1-K38A proteins remain soluble in the supernatant. In the presence of membranes, similar amounts of protein are found in supernatant and pellet with WT protein. However, DLP1-K38A protein shows a high affinity for membranes with most of the protein found associated with the membrane pellet. (B) Densitometric analyses was performed with the use of three separate membrane-pelleting assays. In the absence of membranes, both WT and DLP1-K38A proteins are found in the supernatant (□). Addition of membranes to the reaction mixture results in a substantial increase of DLP1-K38A protein in the pellet compared with WT (▪). S, supernatant; P, pellet.