Short abstract
Objective
To investigate the effectiveness of intrapancreatic choledochal cyst excision in treating type I choledochal cyst, and increase understanding of the need for thorough surgical management of the disease.
Methods
Primary and secondary (including multiple) surgical cases, treated between 2005 and 2015, were retrospectively analysed, and follow-up data of post-treatment effectiveness to date were reviewed. Differences in curative effects were compared between whole and partial excision of the choledochal cyst.
Results
Out of 350 cases, patients with whole excision of the choledochal cyst (n = 272) experienced no associated symptoms in the long-term (3/272 [1.1%] experienced stomach ache or fever). Patients with partial resection of the choledochal cyst (n = 78) developed associated symptoms, including new cyst, calculus of the bile duct (51/78 [65.4%]), and carcinogenesis (11/78 [14.1%]) in the residual intrapancreatic biliary duct. Post-treatment clinical manifestations were significantly different between patients with partial resection versus whole excision of the choledochal cyst (P<0.05).
Conclusion
Surgical re-excision should be considered in patients with a residual intrapancreatic portion of the choledochal cyst due to prior incomplete surgery, regardless of clinical symptoms.
Keywords: Type I choledochal cyst, intrapancreatic portion of the choledochal cyst, surgical management
Introduction
Choledochal cysts, also known as congenital cystic dilatation of the common bile duct, are congenital dilatations of the extra and/or intrahepatic bile ducts that cause various hepatobiliary and pancreatic disorders.1 Choledochal cyst is a relatively rare disease mainly affecting Asian populations, with two thirds of cases occurring in Japan.2 Choledochal cyst can be categorized into five subtypes – type I–V,3 of which type I choledochal cyst accounts for 67.9% of all subtypes.4 The male to female morbidity ratio is reported to be approximately 1:3 or 1:4.5
With a deepening understanding of choledochal cyst pathogenesis, the treatment concept has evolved from ‘unobstructed bile duct drainage’ to ‘whole excision of the cyst to eliminate carcinogenesis risk’ via ‘realization of biliopancreatic diversion’.6 The choledochal cyst should be wholly excised as much as possible during surgery, particularly the intrapancreatic portion of the cyst.7 Previously, due to insufficient understanding of choledochal cyst carcinogenesis, the main purpose of surgical management of the choledochal cyst was biliopancreatic diversion.3 Most extrahepatic choledochal cysts were only excised generally, leaving a residual portion of dilated pancreatic bile duct in vivo.8
The focus of the present study was type I choledochal cyst, which is the most common type observed in the clinic. The present paper is based on retrospective analyses of treatment features in primary and secondary (including multiple surgeries) surgical management of type I choledochal cyst, performed in the Department of General Surgery, Eastern Hepatobiliary, Surgical Hospital affiliated to the Second Military Medical University, Shanghai, China between 2005 and 2015. The aims of the study were to investigate and discuss cases of surgical excision of the intrapancreatic portion of the choledochal cyst, then to compare outcomes between patients who underwent whole excision of the choledochal cyst and those who underwent excision of a portion of the choledochal cyst, and subsequent excision of the residual intrapancreatic portion of the choledochal cyst to reduce the possibility of secondary calculus or carcinogenesis risk, and ensure thorough division of the bile and the pancreatic juice.
Patients and methods
Study population
In this retrospective study, all patients who had received surgical treatment for type I choledochal cyst at the Department of General Surgery, Eastern Hepatobiliary, Surgical Hospital affiliated to the Second Military Medical University, Shanghai, China during 10 years between 2005 and 2015 were included for analysis. All cases were selected according to the diagnosis standard for this disease, and all surgical specimens were verified as choledochal cyst by pathological examination of the excised tissue.9 There were no other inclusion or exclusion criteria.
The study was performed in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Surgical Hospital affiliated to the Second Military Medical University, Shanghai, China. All study participants provided verbal informed consent.
Statistical analyses
Data are presented as n (%) prevalence or mean ± SD and were statistically analysed using SPSS software, version 19.0 (SPSS Inc., Chicago, IL, USA). Differences in post-surgery symptoms (such as stomach ache, fever, jaundice, pancreatitis, residual calculus of the bile duct and carcinogenesis of the residual bile duct) between whole and partial excision of the choledochal cyst were assessed using χ2-test. A P-value < 0.05 was considered statistically significant.
Results
A total of 350 congenital type I choledochal cyst surgical procedures, performed on children and adults, were included in the present study: comprising 272 patients who underwent whole excision of the choledochal cyst (study group A) and 78 patients who underwent excision of the portion of the choledochal cyst (study group B) (Figure 1). Study group A (n = 272) comprised 104 male patients and 168 female patients (median age, 32 years; range, 12–56). Among the 78 patients in group B, 26 were male and 52 were female, with a mean age of 33.6 ± 8.26 years, range 11–52. There were no statistically significant differences in age between male and female patients (P >0.05). All patients included in the study had experienced ≥1 choledochal cyst surgical procedure, with eight patients having undergone two surgical procedures, and one patient having undergone three surgical procedures for choledochal cyst.
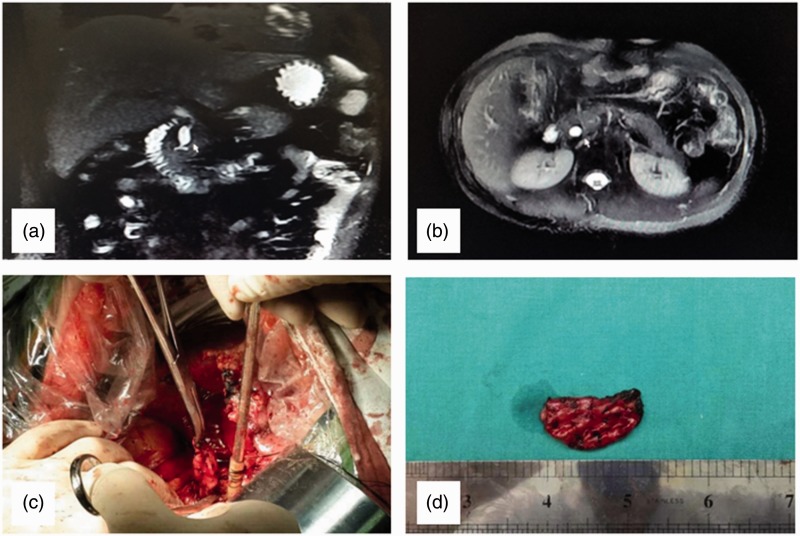
Figure 1.
Representative images of congenital choledochal cyst (type I) surgery showing: (a) Magnetic resonance cholangiopancreatography of partial excision of the choledochal cyst (nonexcision of intrapancreatic bile duct); (b) Magnetic resonance imaging T2 weighted image of a residual intrapancreatic bile duct cyst; (c) Residual intrapancreatic bile duct cyst during surgery; and (d) Specimen of a residual intrapancreatic bile duct cyst: Computed tomography images indicated that acute inflammatory changes of the pancreas were slight
For 78 patients with a residual intrapancreatic portion of the choledochal cyst (group B), postoperative major clinical manifestations were stomach ache (100% [78/78]), fever (41.0% [32/78]) and jaundice (3.8% [3/78]) (Table 1). Although these patients had a residual intrapancreatic portion of the choledochal cyst, most of the symptoms were associated with hepatolithiasis (23.1% [18/78]), original bilio-enteric anastomotic stenosis (51.3% [40/78]), infection of bile duct or other bile duct diseases (44.9% [35/78]). Anomalous haematuria accompanied by increased blood amylase (43.6%, 34/78) and abdominal mass (5.1%, 4/78) was associated with the existence of a residual intrapancreatic portion of the choledochal cyst.
Table 1.
Postoperative choledochal cyst-related symptoms in patients who had undergone whole (group A) or partial (group B) excision of type I choledochal cyst
| Symptom |
Patient group |
χ2value | Statistical significance | |
|---|---|---|---|---|
| A(n = 272) | B (n = 78) | |||
| Stomach ache | 1 | 78 | 344.30 | P <0.05 |
| Jaundice | 0 | 3 | 10.55 | P <0.05 |
| Fever | 2 | 32 | 112.19 | P <0.05 |
| Secondary pancreatitis | 0 | 10 | 35.90 | P <0.05 |
| Residual calculus of bile duct | 0 | 51 | 208.18 | P <0.05 |
| Carcinogenesis of residual bile duct | 0 | 11 | 39.60 | P <0.05 |
Data presented as n patient prevalence.
Statistically significant between-group difference at P <0.05 (χ2-test).
Among patients with residual intrapancreatic portion of the choledochal cyst (group B), secondary calculus was observed in 51/78 patients. The mean time for calculus formation following surgery was 7.1 ± 3.5 years (range 4.5–9 years). The calculus had a white and transparent or semitransparent appearance, which was analogous to pancreatolithiasis, and the texture was crispy or hard with a few of protein clots.
In group B, secondary acute pancreatitis associated with the residual intrapancreatic portion of the choledochal cyst occurred in 10 patients (Table 1) and acute oedematous pancreatitis occurred with mild symptoms, manifesting as pain in the middle and upper abdomen, fever, and haematuria accompanied by increased blood amylase. Computed tomography (CT) images showed that changes in acute inflammation of the pancreas were slight. Extrahepatic bile duct residual calculus was found to be closely associated with secondary pancreatitis. In six of the 10 patients with secondary acute pancreatitis, the calculus existed simultaneously in the intrapancreatic bile duct. Furthermore, of the 10 patients with secondary acute pancreatitis, seven had no clinical history of acute pancreatitis or anomalous haematuria with increased blood amylase, and three had no history of any pancreatitis attack prior to choledochal cyst surgery. In patients who had already experienced postoperative pancreatitis, subsequent attacks were associated with more severe symptoms.
In group B, a total of 11 patients (mean age, 60 years) developed cancer of the residual intrapancreatic portion of the choledochal cyst (Table 1) at between four years (one patient) and 11 years (one patient) post-surgery, with a mean time to cancer diagnosis of eight years. Two of these 11 patients were also found to have calculus in the cancerous bile duct. No clinical symptoms were reported prior to cancer diagnosis. Four patients with secondary carcinogenesis of the bile duct underwent palliative tumour excision, and seven patients underwent pancreaticoduodenectomy excision. Post-hospital outpatient service and telephone follow-up records showed that five patients had died and six patients remained alive to date, with the longest follow-up duration of eight years. One case of bile duct cancer was found during surgery, and at seven years following pancreaticoduodenectomy, no tumour recurrence has been observed in this patient.
Excision of the intrapancreatic portion of the choledochal cyst was completed for 67/78 patients who had not developed cancer of the residual choledochal cyst. Following surgical treatment, these patients have survived to date, without any particular associated symptoms.
Out of 350 cases of whole excision of the choledochal cyst (including primary and multiple surgeries), the junctions of the cholangio-pancreatic duct of most patients with cystic dilatation of the intrapancreatic bile duct were found to be type B-P, with a small piece of stenosis ring existing in the general region prior to the junction of the bile and pancreatic ducts. In 350 cases of type I choledochal cyst surgeries, only one case was found to have a Ø2 mm stomium on the side wall of the pancreatic duct. In this case a supporting conduit was placed into the pancreatic duct, into the duodenum, with an atraumatic line for repairing the pancreatic duct stomium.
Discussion
The present study focused on type I choledochal cyst, the most common subtype of this disease.4 Choledochal cyst presenting with the classical triad of abdominal pain, lump, and jaundice is seen in only 6% of cases, and choledochal cysts with unusual presentations (gastric outlet obstruction, cyst perforation, giant cystolithiasis, giant cyst, and mixed type) have also been reported.10 Cyst excision with hepaticojejunostomy is the standard treatment worldwide, and with increasing clinical practice and study, surgical management of congenital choledochal cyst had greatly improved.7 Various cyst drainage surgeries have higher operative complications and carcinogenesis rates, thus, surgery methods involving excision of the choledochal cyst and bilioenteric anastomosis are important, and have latterly been regarded as first-line treatment methods.11,12 Currently, whole excision of the extrahepatic choledochal cyst is emphasized for primary or secondary surgical treatment.7,13
In China during the 1980’s, surgical treatment generally involved excising most, but not all, of the extrahepatic choledochal cyst.14 The intrapancreatic portion of the choledochal cyst was generally burned by iodine tincture or carbolic acid, or purse string suture was performed at the broken end and the intima was destroyed by curettage and aspiration. Such methods resulted in a residual intrapancreatic portion of the choledochal cyst in vivo, and potential risk of future complications.15 To date, there are no unified viewpoints on whether the residual intrapancreatic portion of the choledochal cyst requires excision when there are no clinical symptoms, and the optimum timing of such surgery remains unclear. To the best of the present authors’ knowledge, there are few published studies regarding surgical excision of the residual intrapancreatic portion of a choledochal cyst.
Four main factors account for reoperation of the choledochal cyst, namely, misdiagnoses, severe associated preoperative diseases, severe complications, or inappropriate surgical procedures. Complete excision of the cyst plus Roux-en-Y hepaticojejunostomy is the ideal surgical strategy.6 The present data of 78 cases indicated that a residual intrapancreatic portion of the choledochal cyst had the potential for multiple pathological changes, such as secondary calculus of the bile duct and pancreatitis, and even carcinogenesis of the bile duct.16,17
In the present study, 51 out of 78 patients who underwent partial resection of the choledochal cyst were found to have secondary calculus of the residual intrapancreatic portion of the cyst, with the fastest time to calculus formation being 1 year following primary surgery. The authors speculate that the mechanism of calculus formation may be related to the existence of an anomalous junction of the pancreatobiliary duct, pancreatic juice regurgitation into the bile duct, stenosed common orifices of the inferior common bile duct or pancreatobiliary duct, and residual bile duct mucosa still possessing some secretory function, plus other factors.
Acute pancreatitis was often secondary to postoperative residual intrapancreatic portion of the choledochal cyst.7 In the present treatment group, secondary acute pancreatitis occurred in 10 patients, seven of whom had no clinical history of acute pancreatitis or anomalous increase in haematuria with increased blood amylase, and three of whom had no history of any pancreatitis attack prior to choledochal cyst surgery. In patients who had already experienced postoperative pancreatitis, subsequent attacks were associated with more severe symptoms. This secondary acute pancreatitis may be related to an anomalous junction of the pancreatobiliary duct, stenosed common orifices of the pancreatobiliary duct, and inadequate drainage of the endocrine in the residual cavity of the bile duct, and regurgitation of the pancreatic duct. Calculus in the residual intrapancreatic portion of the choledochal cyst appeared to be an important inducement for acute pancreatitis attack: Among the above-mentioned 10 patients, six presented with residual calculus of the choledochal cyst intrapancreatic portion. The calculus may have exacerbated inadequate drainage in the residual cavity and led to infection affecting the pancreas.
A residual intrapancreatic portion of the choledochal cyst may lead to secondary carcinogenesis of the bile duct and associated morbidity.18 In the present study, carcinogenesis of the choledochal cyst occurred in 11 patients, with the earliest cancer diagnosis just four years following initial surgery. The mean age of these 11 patients was 60 years, and notably, no clinical symptoms were reported prior to cancer diagnosis, and only two patients had calculus formed in the residual choledochal cyst. These data suggest that calculus was not the sole stimulus of carcinogenesis, and some carcinogenesis factors may exist in the residual intrapancreatic portion of the choledochal cyst, perhaps related to the regurgitation of pancreatic juice into the residual bile duct cavity. Such events may result in mutation of KRAS proto-oncogene, GTPase, anomalous cell proliferation, P53 overexpression, and stimulation of pancreatic juice.19 These results indicate a necessity to surgically excise the asymptomatic residual choledochal cyst.
Diagnosing a residual intrapancreatic portion of the choledochal cyst is relatively easy using type-B ultrasound, CT and magnetic resonance cholangiopancreatography (MRCP).20 Preoperative MRCP examination is indispensable to avoid damaging the pancreatic duct during surgery, as the specific morphology of the cholangio-pancreatic duct junction can be clearly shown.21 Different angles of cholangio-pancreatic duct hydrography can reveal the crossing angle of the cholangio-pancreatic duct junction, rough flow direction of the main pancreatic duct, and the length of the stenosed portion of the inferior bile duct.22 When the imageology data are read, it can be particularly noted that the cyst wall is uniform and smooth, with soft tissue neoplasm inside the cyst wall.23 Peripancreatic lymphonodus was classified as anomalous swelling, so as to avoid the misdiagnosis of carcinogenesis of residual choledochal cyst.24 In this respect, spiral CT should be enhanced due to having a higher image resolution than MRCP and type-B ultrasonography, and the three diagnosis methods may be used together. Serum carcinoembryonic antigen and carbohydrate antigen 19-9 should be listed for routine preoperative examination in choledochal cyst, and be used as important reference indices to estimate carcinogenesis of the bile duct.25
Generally, the position of the pancreatic cyst was in the head of the pancreas, near the back of the pancreas and to the left of the bile duct.26 Separation and searching for the residual intrapancreatic bile duct should begin from the right side behind the head of the pancreas, then gradually go deep into the left side, and the direction of the bile duct should be repeatedly confirmed by fine needle aspiration or finger touch.27 When searching the intrapancreatic bile duct, attention should be paid to stripping of the residual bile duct, and separation should be close to the bile duct wall. When the lumen abruptly tapers near the end of the bile duct, particularly note should be taken of the pancreatic and bile duct jointing here. The present authors have successfully completed more than 300 cases of choledochal cyst whole excision (primary and multiple surgeries) and found that the junctions of the cholangio-pancreatic duct of most patients with cystic dilatation of the intrapancreatic bile duct were type B-P, with a small piece of stenosis ring existing in the general region prior to the junction of the bile and pancreatic ducts. The diameter of the bile duct was only 1–2 mm. If the bile duct was excised, whole excision of the intrapancreatic portion of the choledochal cyst had to be completed.28
In more than 300 cases of type I choledochal cyst surgeries, only one case was found to have a Ø2 mm stomium on the side wall of the pancreatic duct. In this case, a supporting conduit was placed into the pancreatic duct, into the duodenum, with an atraumatic line for repairing the pancreatic duct stomium. There have been no particular associated symptoms in this patient for over six years following surgery. To prevent pancreatic leakage, the wound surface of the pancreas should be closed with a simple interrupted varus suture for no residual cavity. (In recent years, the present surgical group has used omentum tissue to stuff the pancreas residual cavity and has achieved good results.) At the same time, the excised residual bile duct wall should be carefully and conventionally inspected. If cancer was suspected in the anomalous intumescence or jelly-like substance in the bile duct, a pathological examination of frozen tissue section should be performed without delay. If cancer was confirmed, pancreaticoduodenectomy was the sole possible therapeutic method for radical treatment. In the present study, one case of bile duct cancer was found during surgery, and no tumour recurrence has been observed during seven years following pancreaticoduodenectomy.
To date, many hospitals continue to perform partial excision of the cyst with biliary intestinal anastomosis to treat type I choledochal cyst, leaving significant potential risk of future complications. Such surgical methods should be abandoned, and the concept of whole excision of the choledochal cyst should be emphasized. In patients with a residual intrapancreatic portion of the choledochal cyst due to prior incomplete surgery, surgical re-excision should be considered regardless of any accompanying clinical symptoms.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Liu Y., Yao X., Li S., et al. Comparison of therapeutic effects of laparoscopic and open operation for congenital choledochal cysts in adults. Gastroenterol Res Pract 2014; 2014: 670260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Neill JA., Jr Choledochal cyst. Curr Probl Surg 1992; 29: 361–410. [DOI] [PubMed] [Google Scholar]
- 3.Todani T, Watanabe Y, Narusue M, et al. Congenital bile duct cysts: classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg 1977; 134: 263–269. [DOI] [PubMed] [Google Scholar]
- 4.She WH, Chung HY, Lan LC, et al. Management of choledochal cyst: 30 years of experience and results in a single center. J Pediatr Surg 2009; 44: 2307–2311. [DOI] [PubMed] [Google Scholar]
- 5.Liu CL, Fan ST, Lo CM, et al. Choledochal cysts in adults. Arch Surg 2002; 137: 465–468. [DOI] [PubMed] [Google Scholar]
- 6.You Y andGong JP.. Diagnosis and management experience of adult choledochal cysts: reasons for reoperation. Hepatogastroenterology 2013; 60: 470–474. [DOI] [PubMed] [Google Scholar]
- 7.Ando H, Kaneko K, Ito T, et al. Complete excision of the intrapancreatic portion of choledochal cysts. J Am Coll Surg 1996; 183:317–321. [PubMed] [Google Scholar]
- 8.Park SW, Koh H, Oh JT, et al. Relationship between anomalous pancreaticobiliary ductal union and pathologic inflammation of bile duct in choledochal cyst. Pediatr Gastroenterol Hepatol Nutr 2014; 17: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Visser BC, Suh I, Way LW, et al. Congenital choledochal cysts in adults. Arch Surg 2004; 139: 855–860. [DOI] [PubMed] [Google Scholar]
- 10.Gupta N, Gupta V, Noushif M, et al. Unusual presentations of choledochal cyst: case series and review of literature. Indian J Surg 2015; 77: 1318–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singhavejsakul J andUkarapol N.. Choledochal cysts in children: epidemiology and outcomes. World J Surg 2008; 32: 1385–1388. [DOI] [PubMed] [Google Scholar]
- 12.Tan SS, Tan NC, Ibrahim S, et al. Management of adult choledochal cyst. Singapore Med J 2007; 48: 524–527. [PubMed] [Google Scholar]
- 13.Mabrut JY, Bozio G, Hubert C, et al. Management of congenital bile duct cysts. Dig Surg 2010; 27: 12–18. [DOI] [PubMed] [Google Scholar]
- 14.Ko JW, Choi SH, Kwon SW, et al. Robot-assisted hepatectomy and complete excision of the extrahepatic bile duct for type IV-A choledochal cysts. Surg Endosc 2016; 30: 5626–5627. [DOI] [PubMed] [Google Scholar]
- 15.Wang QW, Liu W, Li QL, et al. A modified surgical procedure for intrapancreatic section of choledochal cyst (with a report of 12 cases). Chinese Journal of Modern Operative Surgery 2010-02 [In Chinese, English abstract].
- 16.Wang WJ, Lv ZD, Yang ZC, et al. Prrx1 enhanced the migration ability of breast cancer cell by initiating epithelial-mesenchymal transition. Cancer Cell Res 2017; 4: 362–367. [Google Scholar]
- 17.Chen TT, Liu YQ, Lin BD, et al. Effects of RNA interference of the eno1 gene on the malignant biological behaviors of gastric cell lines. Cancer Cell Res 2017; 4: 326–333. [Google Scholar]
- 18.Dhupar R, Gulack B, Geller DA, et al. The changing presentation of choledochal cyst disease: an incidental diagnosis. HPB Surg 2009; 2009: 103739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwasaki Y, Shimoda M, Furihata T, et al. Biliary papillomatosis arising in a congenital choledochal cyst: report of a case. Surg Today 2002; 32: 1019–1022. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu T, Suzuki R, Yamashiro Y, et al . Magnetic resonance cholangiopancreatography in assessing the cause of acute pancreatitis in children. Pancreas 2001; 22: 196–199 . [DOI] [PubMed] [Google Scholar]
- 21.Yoon JH . Magnetic resonance cholangiopancreatography diagnosis of choledochal cyst involving the cystic duct: report of three cases. ; : –. . Br J Radiol. 2011;84:e18. [Google Scholar]
- 22.Jara H , Barish MA , Yucel EK , et al. MR hydrography: theory and practice of static fluid imaging. ; : –. . AJR Am J Roentgenol. 1998;170:873. [Google Scholar]
- 23.Shindo K , Aishima S , Ohuchida K , et al. Podoplanin expression in the cyst wall correlates with the progression of intraductal papillary mucinous neoplasm. ; : –. . Virchows Arch. 2014;465:265. [Google Scholar]
- 24.Gadelhak N Shehta A andHamed H.. Diagnosis and management of choledochal cyst: 20 years of single center experience . World J Gastroenterol 2014; 20: 7061–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W , Liu Q , Tan SY , et al. Association between carcinoembryonic antigen, carbohydrate antigen 19-9 and body mass index in colorectal cancer patients. ; : –. . Mol Clin Oncol. 2013;1:879. [Google Scholar]
- 26. Ryu M, Takayama W, Watanabe K, et al. Ventral pancreatic resection for adenoma and low-grade malignancies of the head of the pancreas. Surg Today 1996; 26: 476–481. [DOI] [PubMed] [Google Scholar]
- 27.Kolb A, Kleeff J, Frohlich B, et al. Resection of the intrapancreatic bile duct preserving the pancreas. J Hepatobiliary Pancreat Surg 2009; 16: 31–34. [DOI] [PubMed] [Google Scholar]
- 28.Zhang YJ, Qian GX, Zhang BH, et al. An analysis of 45 cases of congenital choledochal cysts undergoing multiple operations. Chinese Journal of Hepatobiliary Surgery 2000; 6: 409–412. [Google Scholar]