Abstract
Peripheral chemoreflex mediated increases in both parasympathetic and sympathetic drive under chronic hypoxia may evoke bradyarrhythmias during apneic periods. We determined whether 1) voluntary apnea unmasks arrhythmia at low (344 m) and high (5,050 m) altitude, 2) high-altitude natives (Nepalese Sherpa) exhibit similar cardiovagal responses at altitude, and 3) bradyarrhythmias at altitude are partially chemoreflex mediated. Participants were grouped as Lowlanders (n = 14; age = 27 ± 6 yr) and Nepalese Sherpa (n = 8; age = 32 ± 11 yr). Lowlanders were assessed at 344 and 5,050 m, whereas Sherpa were assessed at 5,050 m. Heart rate (HR) and rhythm (lead II ECG) were recorded during rest and voluntary end-expiratory apnea. Peripheral chemoreflex contributions were assessed in Lowlanders (n = 7) at altitude after 100% oxygen. Lowlanders had higher resting HR at altitude (70 ± 15 vs. 61 ± 15 beats/min; P < 0.01) that was similar to Sherpa (71 ± 5 beats/min; P = 0.94). High-altitude apnea caused arrhythmias in 11 of 14 Lowlanders [junctional rhythm (n = 4), 3° atrioventricular block (n = 3), sinus pause (n = 4)] not present at low altitude and larger marked bradycardia (nadir −39 ± 18 beats/min; P < 0.001). Sherpa exhibited a reduced bradycardia response during apnea compared with Lowlanders (P < 0.001) and did not develop arrhythmias. Hyperoxia blunted bradycardia (nadir −10 ± 14 beats/min; P < 0.001 compared with hypoxic state) and reduced arrhythmia incidence (3 of 7 Lowlanders). Degree of bradycardia was significantly related to hypoxic ventilatory response (HVR) at altitude and predictive of arrhythmias (P < 0.05). Our data demonstrate apnea-induced bradyarrhythmias in Lowlanders at altitude but not in Sherpa (potentially through cardioprotective phenotypes). The chemoreflex is an important mechanism in genesis of bradyarrhythmias, and the HVR may be predictive for identifying individual susceptibility to events at altitude.
NEW & NOTEWORTHY The peripheral chemoreflex increases both parasympathetic and sympathetic drive under chronic hypoxia. We found that this evoked bradyarrhythmias when combined with apneic periods in Lowlanders at altitude, which become relieved through supplemental oxygen. In contrast, high-altitude residents (Nepalese Sherpa) do not exhibit bradyarrhythmias during apnea at altitude through potential cardioprotective adaptations. The degree of bradycardia and bradyarrhythmias was related to the hypoxic ventilatory response, demonstrating that the chemoreflex plays an important role in these findings.
Keywords: apnea, arrhythmia, chemoreflex, hypoxia, Sherpa
INTRODUCTION
It has been traditionally shown that efferent sympathetic and vagal outflow to the heart is reciprocal, through which increased activation of one pathway sees a respective decrease in the other (17). As such, the variation of cardiac sinus conduction is controlled through the balance between neural outflows in healthy populations. Concurrent increases in both pathways can occur under specific circumstances and have previously been referred to as cardiac “autonomic conflict” (29). This has previously been reported during periods of considerable autonomic stress (e.g., cold-water submersion) due to conflicting activation of both sympathetic and parasympathetic pathways, ultimately promoting cardiac arrhythmogenesis in healthy individuals (9, 33). In addition, conflict can be seen to some degree in clinical populations suffering from sleep apnea, where elevated chemoreflex gain during apneic periods is linked to both hypertensive and bradycardia responses (24, 30). However, the degree of conflict can be considered minimal, with no previous accounts of arrhythmogenesis being noted in healthy populations exhibiting normal chemoreflex function during apnea (e.g., volitional breath holding).
Heightened chemoreflex activity under chronic hypoxia results in a concurrent increase of efferent peripheral sympathetic nerve activity and cardiac vagal tone (8). However, these increases are normally dampened by the inhibitor influence of pulmonary stretch receptors, ultimately blunting sympathetic outflow to heightened chemoreflex stress (19, 28, 31). As such, there is limited electrocardiographic evidence that suggests chronic hypoxia exposure leads to incidences of bradycardic arrhythmia within healthy individuals during sleep (4). The peripheral chemoreflex has been implicated specifically in these cases as the changes in heart rate observed appear correlated to the ventilatory response to acute hypoxia (22). Thus there is a mechanistic basis for hypothesizing that chemoreflex sensitization during acclimatization at altitude (10) may promote autonomic conflict and potential bradycardic arrhythmic events during periods of sleep-related apnea. The use of voluntary apnea during chronic altitude exposure is therefore a relevant experimental model to investigate autonomic cardiac function independent of underlying comorbidities.
Recently, we conducted a high-altitude research expedition to 5,050 m in the Himalayan mountain range of Nepal. Our goal was to study autonomic function during chronic hypoxic exposure in Lowlanders. We contrasted these data with high-altitude natives (Nepali Sherpa) to examine whether they would have similar vagal drive as Lowlanders despite generations of residency at altitude. Our study was designed to investigate experimentally 1) whether voluntary apnea would unmask vagally mediated bradycardia or conduction abnormalities during wakefulness at altitude (5,050 m), 2) the degree to which the peripheral chemoreflex plays a role in the susceptibility to bradycardic arrhythmia at altitude, and 3) whether low- and high-altitude natives exhibit similar cardiovagal responses to apnea. Based on previous findings, we hypothesized that voluntary apnea in the awake state would experimentally unmask heightened vagal activity and indications of autonomic conflict in Lowlanders at altitude, characterized by bradycardia and arrhythmias.
METHODS
Study participants.
Fourteen Lowlanders (two women; age = 27 ± 6 yr) and eight highland Nepalese Sherpa (age = 32 ± 11 yr) from the Khumbu region of Nepal participated after providing informed, written consent. All procedures were explained in English and Nepalese and approved by the University of Alberta Biomedical Research Ethics Board, the University of British Columbia Clinical Research Ethics Board, and the Nepal Health Research Council (Pro00064195) in compliance with the Declaration of Helsinki. Health-history screening was negative for any pre-existing cardiovascular, respiratory, or neurological disorders. Four Sherpa were current smokers (0.42 ± 0.7 pack yr).
Resting baseline and apnea protocol.
Pre-expedition testing (Lowlanders) was conducted at 344 m (Kelowna, Canada). After flying to 2,840 m, participants followed a conservative ascent profile (9–10 days) to the Ev-K2-CNR research facility (5,050 m; Nepal). Two Lowlanders were administered medication for treatment of altitude illness during ascent [a single dose of acetazolamide (125 mg) or dexamethasone] but were tested following a minimum 48-h washout period. Sherpa were tested on days 1–4, and Lowlanders were tested after 4–10 days at 5,050 m. Both Sherpa and Lowlanders exhibited similar resting peripheral oxygen saturation (SpO2; 82 and 83%, respectively) at 5,050 m.
Participants were tested in the supine position. ECG (lead II) and arterial blood pressure (finger photoplethysmography; Finometer PRO; Finapres Medical Systems) were collected continuously at 1 kHz (Chart Pro 8.3.1; ADInstruments). Brachial arterial pressure waveform was back-calibrated through return-to-flow correction confirmed against manual brachial measurements. Mean (MAP), systolic (SBP), and diastolic (DBP) pressures were calculated on a beat-by-beat basis from the calibrated pressure waveform. Beat-by-beat cardiac output (CO) was calculated using the Model Flow algorithm and used to calculate total peripheral resistance (TPR = MAP/CO). SpO2 was continually assessed (pulse oximetry; Nellcor; Medtronic). Following 10 min of quiet baseline measures, participants were instructed to perform an end-expiratory apnea (at functional residual capacity). An investigator paced participants’ breathing and signaled when to initiate apnea. Participants wore a nose clip and were instructed to “hold their breath for as long as possible.” The role of the peripheral chemoreceptors was assessed in seven Lowlanders by repeating apnea at altitude after 1–2 min of prebreathing 100% oxygen. In addition, individual hypoxic ventilatory responses (HVRs; Δventilation/ΔSpO2) at altitude were recorded in a subset of Lowlanders within our study (n = 11; 1 woman) and Sherpa (n = 6) by breathing a fixed fraction of inspired oxygen (~16%) for 5 min while at 5,050 m. However, this HVR was only measured once and not during successive periods while at altitude. We were unable to obtain the HVR in 3 of the 14 Lowlanders and 2 of the 8 Sherpa. These HVR measures were performed independently of the study, although they were at similar time points.
Data and statistical analysis.
To determine whether voluntary apnea unmasks autonomic conflict in Lowlanders and Sherpa at altitude, values were calculated at two periods within each group (Lowlanders, Lowlanders with supplemental oxygen, and Sherpa) and condition (low and high altitude). Baseline values were calculated over 5 min of spontaneous breathing. Electrophysiological characteristics (waveform amplitudes, durations, and intervals) of the ECG were assessed during the 30 s immediately preceding apneas; cardiac cycles (15–45) were overlaid, aligned with the R wave, and the aggregate was analyzed using automated software (Chart Pro 8.3.1). To account for variation in apnea duration, cardiovascular data from the final 10 cardiac cycles of each apnea were analyzed. A cardiologist (S. van Diepen) identified and classified conduction abnormalities from ECG waveforms from the three beats immediately preceding and three beats following apnea breakpoint.
Baseline heart rate variability (HRV) was calculated during 5 min to contrast the relative contribution in sympathetic and parasympathetic activation between low and high altitude and under supplemental oxygen at high altitude. HRV was analyzed using commercially available software (MLS310/8 HRV; ADInstruments). Frequency-domain analyses included spectrum analysis of very low (VLF; 0–0.04 Hz), low (LF; 0.04–0.15 Hz), and high (HF; 0.15–0.40 Hz) frequency bands. Temporal domain analyses included the standard deviations between successive NN intervals (SDNN) and root mean square of the successive differences (RMSSD). Total power was calculated as the variance of all NN intervals. The ratio of LF to HF power (LF/HF) was also used.
Statistical analyses were performed using SigmaStat 3.13 (Systat Software, Chicago, IL). Results are reported as means ± standard deviation. Differences in cardiovascular data between conditions (low vs. high vs. high + oxygen) and between groups (Lowlanders vs. Sherpa) were assessed using preplanned contrasts (paired and unpaired t-tests). Mann-Whiney tests were run in incidences of nonnormal distributions. Differences in incidence of arrhythmias between conditions in Lowlanders were assessed using McNemar test for paired dichotomous data. To correct for multiple comparisons (c), the a priori α (0.05) was adjusted (α′) using the experimentwise error rate (αe; Refs. 15, 32):
Relationships between measures were assessed using Pearson correlations. Receiver operating characteristic (ROC) curve analysis was performed to assess the specificity and sensitivity of an individual’s HVR to predict the susceptibility to arrhythmia during apnea at altitude.
RESULTS
All 14 Lowlanders were successfully tested at 344 and 5,050 m. No sex differences were present between the 2 women in the Lowlander group. Before testing, 1 Lowlander was categorized as having mild acute mountain sickness at altitude (Lake Louise Score of 3), but no other participants exhibited symptoms of illness.
Group characteristics and cardiovascular function for each condition are reported in Table 1. HR increased in Lowlanders at high altitude (P < 0.05 vs. low altitude), becoming similar to Sherpa. SBP, DBP, MAP, CO, and TPR were unchanged in Lowlanders at high altitude (SBP, P = 0.060; DBP, P = 0.782; MAP, P = 0.901; CO, P = 0.159; and TPR, P = 0.056) and no different from Sherpa (SBP, P = 0.786; DBP, P = 0.287; MAP, P = 0.641; CO, P = 0.581; and TPR, P = 0.789). High-altitude HVR was not different between Lowlanders (1.11 ± 1.78 l·min−1·%desaturation−1) and Sherpa (0.28 ± 0.16 l·min−1·%desaturation−1; P = 0.317). This remained nonsignificant even after accounting for one apparent Lowlander “outlier” with a high HVR (6.2 l·min−1·%desaturation−1; P = 0.242).
Table 1.
Demographic and cardiovascular function in Lowlanders and Sherpa at low and high altitudes
| Lowlanders |
Sherpa |
|||
|---|---|---|---|---|
| 344 m (n = 14) | 5,050 m (n = 14) | 5,050 m + Oxygen (n = 7) | 5,050 m (n = 8) | |
| Subject demographics | ||||
| Age, yr | 27 ± 6 | 27 ± 6 | 30 ± 8 | 32 ± 13 |
| Height, m | 1.77 ± 0.8 | 1.77 ± 0.8 | 1.79 ± 0.06 | 1.68 ± 0.08 |
| Weight, kg | 72.2 ± 10.1 | 69.4 ± 8.6 | 69.3 ± 10.3 | 63.7 ± 10.1 |
| Body mass index, kg/m2 | 23.1 ± 2.8 | 22.2 ± 2.5 | 21.5 ± 2.9 | 22.8 ± 3.5 |
| Resting cardiovascular function | ||||
| Heart rate, beats/min | 61 ± 15 | 70 ± 15† | 62 ± 10‡ | 71 ± 5†§ |
| SpO2, % | 98 ± 1 | 82 ± 3† | 96 ± 1‡ | 83 ± 4†§ |
| Systolic pressure, mmHg | 119 ± 9 | 113 ± 13 | 113 ± 8 | 111 ± 9 |
| Diastolic pressure, mmHg | 66 ± 7 | 70 ± 10 | 71 ± 8 | 65 ± 8 |
| Mean pressure, mmHg | 84 ± 8 | 86 ± 11 | 89 ± 7 | 84 ± 9 |
| Cardiac output, l/min* | 5.9 ± 1.8 | 5.5 ± 1.4 | 5.1 ± 1.1 | 6.0 ± 1.7 |
| Total peripheral resistance* | 15 ± 4 | 17 ± 4 | 19 ± 7 | 16 ± 7 |
Values calculated using Model Flow.
Significantly different from Lowlanders tested at low altitude (344 m), P < 0.05.
Significantly different from Lowlanders tested at high altitude (5,050 m), P < 0.05.
Significantly different from Lowlanders during hyperoxia (5,050 m + oxygen), P < 0.05.
Ascent to altitude saw a shortening of the P-R interval (P < 0.001) and P-wave duration (P < 0.05), QRS complex widening (P < 0.001), heart-rate-corrected QT (QTc) prolongation (P < 0.001), and P-wave amplitude depression (P < 0.001) in Lowlanders (Table 2). Sherpa also exhibited widened QRS complexes (P < 0.001) and longer QTc (P < 0.05) at altitude compared with Lowlanders at low altitude (Table 2). The incidence of arrhythmia at rest was low in all groups and conditions (Table 3). One Lowlander exhibited periodic premature ventricular contractions during rest at both altitudes, but the incidence remained low (<2/min) and unchanged between altitudes. On ascent to altitude, one Lowlander developed persistent junctional rhythm that was not relieved with oxygen. No arrhythmias were noted in Sherpa at rest.
Table 2.
Electrocardiogram measurements made in Lowlanders and Sherpa at low and high altitudes during rest and apnea
| Lowlanders |
Sherpa |
|||
|---|---|---|---|---|
| 344 m (n = 14) | 5,050 m (n = 14) | 5,050 m + Oxygen (n = 7) | 5,050 m (n = 8) | |
| Rest | ||||
| P-wave duration, ms | 96 ± 10 | 77 ± 20b | 87 ± 12 | 97 ± 16c |
| P-wave amplitude, mV | 0.16 ± 0.05 | 0.14 ± 0.04 | 0.14 ± 0.06 | 0.11 ± 0.02b,d |
| PR interval, ms | 169 ± 19 | 124 ± 27b | 146 ± 51 | 158 ± 38c |
| QRS duration, ms | 71 ± 14 | 121 ± 3b | 119 ± 6b | 120 ± 1b |
| QTc, msa | 398 ± 26 | 456 ± 28b | 449 ± 23b | 408 ± 15c,d |
| R-wave amplitude, mV | 1.76 ± 0.70 | 1.24 ± 0.60 | 1.49 ± 0.45b | 1.19 ± 0.22b |
| T-wave amplitude, mV | 0.49 ± 0.19 | 0.28 ± 0.14b | 0.39 ± 0.19b | 0.36 ± 0.15b |
| Apnea | ||||
| P-wave duration, ms | 96 ± 20 | 74 ± 27 | 68 ± 22 | 68 ± 34c,d |
| P-wave amplitude, mV | 0.15 ± 0.05 | 0.10 ± 0.06b | 0.10 ± 0.06b | 0.09 ± 0.06b |
| PR interval, ms | 163 ± 31 | 128 ± 26b | 151 ± 55 | 157 ± 37 |
| QRS duration, ms | 74 ± 13 | 119 ± 4b | 117 ± 5b | 113 ± 10b |
| QTc, msa | 398 ± 34 | 410 ± 33e | 417 ± 31b | 465 ± 51d |
| R-wave amplitude, mV | 1.67 ± 0.79 | 1.45 ± 0.74 | 1.65 ± 0.60b | 1.25 ± 0.34 |
| T-wave amplitude, mV | 0.48 ± 0.19 | 0.32 ± 0.18b | 0.40 ± 0.28 | 0.37 ± 0.13 |
All measurements were taken using a standard lead II configuration. Measurements during apnea were taken from the 10 cardiac cycles before volitional breakpoint.
Framingham correction (QT + 0.154 × (1 − RR)].
Significantly different from Lowlanders tested at low altitude (344 m), P < 0.05.
Significantly different from Lowlanders tested at high altitude (5,050 m), P < 0.05.
Significantly different from Lowlanders during hyperoxia (5,050 m + oxygen), P < 0.05.
Significantly different from rest within the same condition/group, P < 0.05.
Table 3.
ECG conduction abnormalities identified at rest and during apnea
| Lowlanders |
Sherpa |
|||
|---|---|---|---|---|
| 344 m (n = 14) | 5,050 m (n = 14) | 5,050 m + Oxygen (n = 7) | 5,050 m (n = 8) | |
| Abnormalities identified at rest | ||||
| Premature ventricular contractions (<2/min) | 1 | 1 | 1 | |
| Junctional rhythm | 1 | 1 | ||
| Abnormalities associated with apnea* | ||||
| Atrial bigeminy | 1 | |||
| Premature atrial contractions | 1 | |||
| Ectopic atrial rhythm | 1 | |||
| Nonconducted sinus beat | 1 | |||
| Nonconducted sinus beat with junctional escape | 1 | |||
| Sinus pause/arrest | 1 | |||
| Sinus pause/arrest with junctional escape | 2 | |||
| Sinus pause with junctional rhythm | 1 | |||
| Sinus arrest with junctional rhythm | 3 | 1 | ||
| 3° Atrioventricular block | 3 | 1 | ||
ECG assessment was carried out by a cardiologist (S. van Diepen) who was blinded to group and condition. Premature ventricular contractions were observed at rest in the same individual under all conditions; the rate of occurrence did not change with condition. One individual developed persistent junctional rhythm at altitude, and this persisted during the oxygen administration.
All conduction abnormalities associated with voluntary apnea occurred immediately preceding or following (<3 beats) breakpoint.
Lowlanders saw no difference in indices of HRV (SDNN, RMSDD, and total power) between low and high altitude with the exception of RR intervals, which became significantly decreased (1,157 ± 240 vs. 979 ± 208 ms; P < 0.001) at high altitude. Oxygen supplementation did not change SDNN, RMSDD, total power, and RR intervals at altitude. Sherpa did not exhibit any difference in HRV measures from Lowlanders at high altitude. For all groups/altitudes, there was no difference in frequencies (VLF, LF, and HF) and total power. However, Lowlanders exhibited a significant increase in the LF/HF ratio at high altitude (P < 0.05), whereas both the supplemental oxygen group and Sherpa LF/HF ratio were not different at altitude.
Responses to voluntary apnea at low altitude.
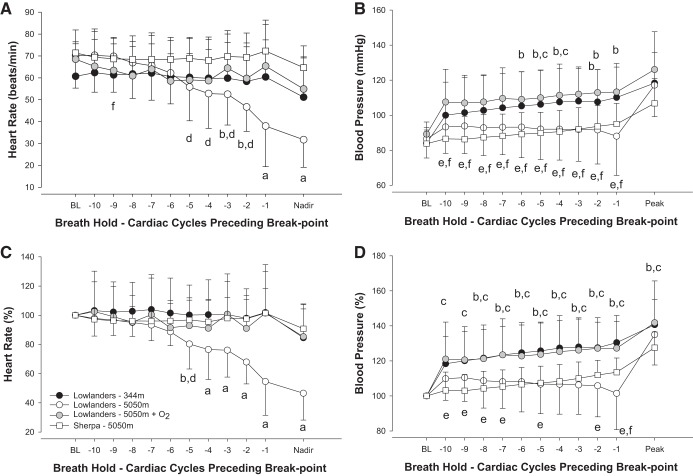
At low altitude, Lowlander SpO2 was 98 ± 1% before apneas but was not obtained during or immediately after apnea. Lowlanders had an apnea duration of 30.4 ± 11.1 s (range 15–74 s), which resulted in a modest bradycardia response (−10 ± 15 beats/min; P < 0.001; Fig. 1). No changes in ECG parameters were noted during apnea at low altitude, although 3 of the 14 Lowlanders developed arrhythmia (premature atrial contractions, atrial bigeminy, and nonconducted sinus beat; Table 3).
Fig. 1.
Responses to apnea in Lowlanders at 344 m (n = 14; black circles), Lowlanders at 5,050 m (n = 14; white circles), Lowlanders + O2 at 5,050 m (n = 7; gray circles), and Sherpa at 5,050 m (n = 8; white squares). A: absolute bradycardia response to apnea. B: absolute pressor response during apnea. C: percentage change of bradycardic response to apnea. A significant bradycardia response was observed in Lowlanders at high (but not) low altitude. Apnea after 100% oxygen eliminated bradycardia in Lowlanders. Sherpa did not exhibit bradycardia during apnea at altitude. D: percentage change of pressor response during apnea. All data have been aligned to breakpoint, and the last 10 cardiac cycles have been plotted. The mean nadir/peak responses are also identified. BL, baseline. aLowlanders at high altitude significantly different from all other groups, P < 0.05; bsignificant difference between Lowlanders at low altitude vs. Lowlanders at high altitude, P < 0.05; csignificant difference between Lowlanders at low altitude and Sherpa, P < 0.05; dsignificant difference between Lowlanders at high altitude and Sherpa, P < 0.05; esignificant difference between Lowlanders + oxygen (n = 7) at altitude and Sherpa, P < 0.05; fsignificant difference between Lowlanders + oxygen and Lowlanders (without oxygen) at altitude, P < 0.05.
Responses to voluntary apnea at high altitude.
At high altitude, resting SpO2 was similar in Lowlanders (82 ± 3%) and Sherpa (83 ± 4%; P = 0.933). Apnea resulted in further desaturation of Lowlanders (nadir 78 ± 7%) and Sherpa (nadir 75 ± 5%; P = 0.344 vs. Lowlanders at altitude). Apnea duration was also reduced in Lowlanders [15.4 ± 5.3 s (range 9–27 s); P < 0.001 compared with low altitude] that was similar to Sherpa (15.8 ± 2.6; range 12–19 s).
Lowlander apneas saw magnified bradycardia at high altitude (−39 ± 18 beats/min; P < 0.001 vs. low altitude). In contrast, Sherpa had a reduced extent of bradycardia during apnea (−7 ± 10 beats/min; P < 0.001 vs. Lowlanders). Despite bradycardia, blood pressure progressively rose in Lowlanders to a peak MAP at low altitude (112 ± 19 mmHg; P < 0.001 vs. baseline) and high altitude (100 ± 21 mmHg; P < 0.01 vs. baseline). Peak blood pressure response was similar in Sherpa (100 ± 12 mmHg; P = 1.035 vs. Lowlanders at altitude; Fig. 1). Between rest and apnea, only QTc duration was significantly reduced (P < 0.01) in Lowlanders. Apneas at high altitude resulted in a prolongation of the QRS (P < 0.01) and PR intervals (P < 0.05), whereas P- and T-wave amplitudes were depressed (P < 0.001) in Lowlanders. Sherpa and Lowlanders exhibited mostly similar ECG values during apnea at altitude. However, Sherpa P-wave duration was significantly prolonged (P < 0.05) compared with Lowlanders.
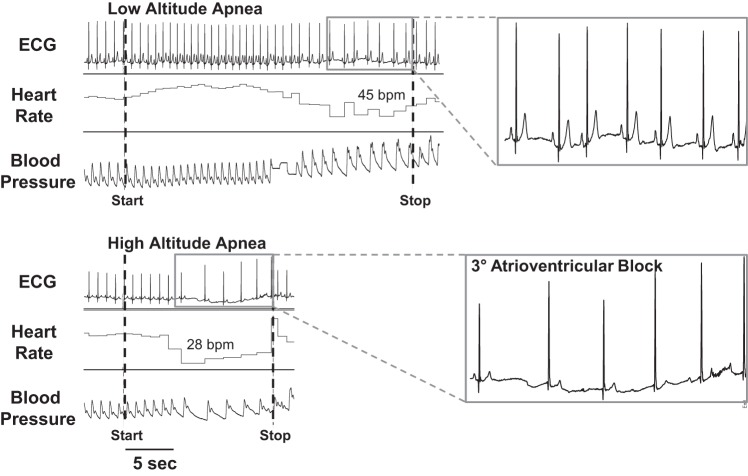
At high altitude, there was a greater incidence in arrhythmias during apnea in Lowlanders (11 of 14; P < 0.05) compared with low-altitude apnea. Identified arrhythmias included sinus pause/arrest, junctional, and 3° atrioventricular block (Fig. 2; Table 3). In contrast, no abnormalities were apparent during apnea in Sherpa despite no differences in the duration of apnea or degree of desaturation compared with Lowlanders at altitude.
Fig. 2.
Raw data demonstrating apnea-induced arrhythmia at altitude. Examples of ECG tracings from the same male participant during apnea at low (top) and high altitudes (bottom). Apnea at altitude exhibited arrhythmic events, such as 3° atrioventricular block (see inset, bottom right). bpm, Beats per minute.
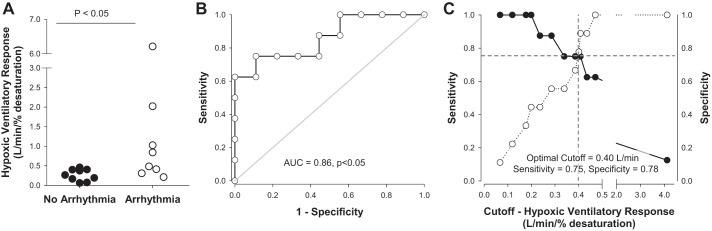
As HVR was similar between groups (see above), including the one female participant, data were combined to assess the relationship between HVR and heart rhythm. The gain of the bradycardic response during apnea across groups was correlated with HVR (Fig. 3). Two Lowlanders were identified as statistical outliers using Studentized residuals; however, the relationship remained significant when these individuals were either included or removed from the analysis (Fig. 3). When data were grouped based on the presence or absence of arrhythmia during apnea, those individuals exhibiting apneas had significantly higher HVRs (median 0.66 l/min) vs. those who did not exhibit arrhythmias (median 0.26 l/min; P < 0.02; Fig. 4A). ROC analysis further indicated that HVR was significantly predictive of the incidence of arrhythmia during apnea (area under the curve = 0.86; P < 0.05; Fig. 4B) with a sensitivity of 75% and specificity of 78% when using an optimized HVR cutoff of 0.40 l/min (Fig. 4C). However, there was no relationship between HVR and the magnitude of bradycardia during apnea (r2 = 0.08).
Fig. 3.
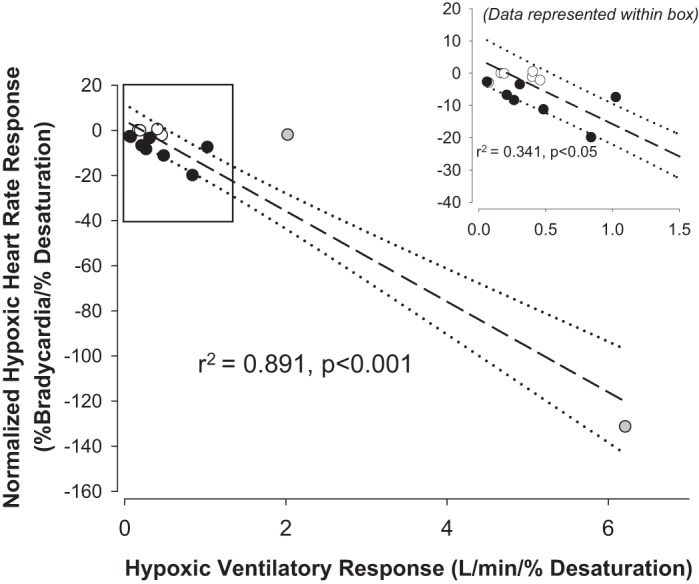
Correlation analysis between the normalized bradycardic response to apnea and hypoxic ventilatory response at altitude across groups. Closed circles represent Lowlanders (n = 11; 1 woman), and open circles represent Sherpa (n = 6). Two Lowlanders were identified at statistical “outliers” (gray symbols) based on Studentized residuals. However, a significant relationship was also maintained if these participants were excluded (inset). The dashed lines and dotted lines represent the linear regressions and 95% confidence intervals, respectively.
Fig. 4.
Hypoxic ventilatory response was higher in individuals who developed arrhythmias during apnea (A). Receiver operating curve analysis indicated that the hypoxic ventilatory response was significantly predictive of the incidence of arrhythmia at altitude (B). AUC, area under the curve. When an optimal cutoff was determined (0.40 l·min−1·%desaturation−1), the hypoxic ventilatory response was predictive of arrhythmias with a sensitivity of 75% and specificity of 78% (C). A: open circles, arrhythmia; black circles, no arrhythmia. B: open circles with solid lines are arbitrary (represent relationship between sensitivity and specificity points). C: closed circles with solid line represent Sensitivity, whereas open circles with dashed line represent Specificity.
Influence of supplemental oxygen to apneic response at high altitude.
Supplemental oxygen was administered to 7 out of the 14 Lowlanders at high altitude before voluntary apnea. This increased initial SpO2 from 82 ± 3 to 96 ± 1% (P < 0.001) before apnea and reduced resting heart rate (62 ± 10 beats/min; P < 0.05 vs. altitude). Apnea duration was prolonged (67.0 ± 45.2 s; P < 0.01 vs. euoxic apnea) following oxygenation. Oxygenation significantly blunted apnea-related bradycardia (Fig. 1; P < 0.05) but increased associated peak in MAP (117 ± 16 mmHg; P < 0.05). Supplementation of oxygen returned R-wave amplitude to low-altitude values, and the incidence of arrhythmia was significantly reduced compared with euoxic apnea (3 of 7 Lowlanders; P < 0.05; Table 3).
DISCUSSION
In the current study, we have demonstrated that through the use of voluntary apnea at altitude, there exists considerable underlying vagal and sympathetic drive in Lowlanders as marked by significant bradycardia and incidence of bradyarrhythmias. In contrast, Sherpa exhibited a less pronounced bradycardia during apnea and an absence of arrhythmias. With the use of supplemental oxygen, we further demonstrated that the augmented bradycardia and arrhythmias observed in Lowlanders were specifically related to the peripheral chemoreflex. This was also supported by a significant relationship between the participant-specific HVR and the degree of bradycardia occurring during apnea. Furthermore, ROC analysis indicated that heightened HVR was significantly predictive of the susceptibility to high-altitude arrhythmias during apnea.
At altitude, Lowlanders exhibited shorter P-wave duration and PR interval as well as enlarged P- and R-wave amplitudes, suggesting an elevated sympathetic drive as seen previously (11, 14). In clinical contexts, shortening of P and PR intervals is associated with increased risk of atrial fibrillation (1, 26). Despite changes in ECG conductance, arrhythmias during baseline were not observed in either Lowlanders or Sherpa. Previous accounts of altitude-related arrhythmias in Lowlanders have been reported. These have been primarily documented during periods of physical exertion after rapid ascent (3) or during sleep (4). Similar to our findings, these events were also associated with flattened T-wave amplitudes and P-wave shortening (3). Recently, Woods et al. (34) noted the presence of symptomatic sinus tachycardia at altitude (n = 2) during periods of strenuous exercise via implantable loop recorder, whereas Boos et al. (4) also noted arrhythmias during periods of exertion at altitude in 16 Lowlanders using continuous ECG monitoring. In both reports, arrhythmia incidence was increased at higher altitudes (5,000–7,550 m) as well as with longer exposure periods (4, 34). Both our data and these previous findings suggest high altitude to be a “proarrhythmia” environment, where the influence of hypoxia may be compounded further during periods of stress. Although we did not obtain continuous ECG monitoring through the study, our findings agree with the presence of arrhythmias during periods of heightened stress.
Previous studies show increased periodic breathing and central apnea at altitude in Lowlanders (2, 5, 27) as well as associated periods of bradycardia (16, 20, 22, 27) and arrhythmia (6, 16, 20). Thus the current study utilized voluntary apnea to characterize the mechanisms of altitude-related bradycardia and arrhythmia in Lowlanders at rest. The significant bradycardia and development of arrhythmias (11 of 14 Lowlanders) during apnea is indicative of heightened sympathetic and parasympathetic innervation of the heart at altitude (8, 17, 25). Although the relationship between sympathetic and parasympathetic control of the heart is often considered reciprocal, conditions where both pathways are concurrently elevated cause the heart to experience what has previously been termed as autonomic conflict. The occurrence of cardiac arrhythmias during cold-water immersion has been attributed to this conflict (29) when high sympathetic (cold shock response) and parasympathetic (mammalian diving reflex) activity occurs. As classically described, the primary cardiovascular consequences of peripheral chemoreceptor activation are concurrently elevated sympathetic and parasympathetic activity (8). However, under eupneic conditions, hypoxia engages pulmonary reflexes (via the hypoxic ventilatory response) that inhibit both parasympathetic (8) and sympathetic activity (19, 28, 31). The degree of chemoreflex sensitivity and its direct relationship to sympathetic augmentation at altitude is not fully understood. Despite this, earlier works have demonstrated that the augmentation of sympathetic activity under acute hypoxia exposure is driven through heightened peripheral chemoreflex activation (23, 35). In the current study, we have demonstrated a similar autonomic conflict that is unmasked during apnea and mediated via the peripheral chemoreflex. First, we showed that the bradycardia associated with apnea at altitude was correlated with the hypoxic ventilatory response. Second, oxygen administration before apnea at altitude eliminated bradycardia and reduced the incidence of arrhythmia to the same level as observed at low altitude.
Although Sherpa had a similar breath-hold duration and resting arterial oxygen saturation, they did not exhibit significant bradycardia or any arrhythmias. Previous data from native Tibetans indicate a normal ECG compared with Han residents who had migrated during childhood to high altitude (13). We saw that breath-holding generated arrhythmias in most Lowlanders but not Sherpa. Thus these findings suggest that Sherpa exhibit an altered cardiac response to hypoxic stress. However, it is unclear what the specific nature of the adaptation is that might be present. We do not believe this is related to differences in chemoreflex sensitivity as our findings are consistent with recent data indicating Sherpa to have similar chemoreflex sensitivity to acclimatized Lowlanders (7, 12, 36). However, when Lowlanders and Sherpa were considered together, we found that high-altitude HVR was significantly related to the normalized bradycardic response to high-altitude apnea. Previously, Masuyama et al. (21) observed a significant relationship between low-altitude HVR and high-altitude sleep-related (normalized) bradycardia. These previous data are intriguing as the relationship they demonstrate is apparent across conditions (low vs. high altitude) and sleep state (awake vs. sleep). This would suggest a robust predictive utility of low-altitude HVR. In keeping with this, we demonstrated that high-altitude HVR was significantly predictive of arrhythmias during apnea at altitude and therefore potentially useful as a predictor for risk of high-altitude arrhythmia.
Considerations.
The current study was a part of a larger research expedition to the Himalayan Range in Nepal and involved several independent studies examining vascular, cerebral autoregulatory, neuromuscular, and autonomic function between Lowlanders and Sherpa. As such, certain time-dependent and technical limitations exist with regard to testing both Lowlanders and Sherpa at altitude. One limitation was the inability to repeat the supplemental oxygen trial within Sherpa. Sherpa were initially tested at 5,050 m and soon followed by Lowlanders. However, significant arrhythmic events were only noted in Lowlanders at 5,050 m. Following several examples of arrhythmias within Lowlanders, we attempted to address the potential chemoreflex contribution through supplemental oxygen. However, Sherpa exhibited neither considerable bradycardic responses nor arrhythmias during apnea. Therefore, we believe that supplemental oxygen would not have produced any considerable difference in cardiovagal responses during apnea.
We demonstrate a relationship between the HVR and degree of bradycardic response at altitude. However, we acknowledge that two individuals with high HVRs (statistically identified as outliers in relation to the rest of our participants) appear to weight this relationship. Nonetheless, even if these two participants were removed, there still existed a significant relationship between HVR and bradycardic response (see Fig. 3). We acknowledge that our measure of HVR may exhibit some degree of ventilatory suppression through respiratory-induced alkalosis, thus further minimizing central chemoreceptor activation. This limitation within our HVR measure should be considered during interpretation of results. Our specific goal was to assess peripheral chemoreceptor contributions within a high-altitude field setting. Because of the nature of the technique that was utilized for determining HVR (measuring the ventilatory response to continuous 16% fraction of inspired oxygen at 5,050 m) without successive measurements, it would be recommended that utilizing HVR for predicting bradycardic events should be investigated further to confirm the present findings.
Conclusion.
Our results suggest increased parasympathetic activity and sympathetic drive at altitude promote autonomic conflict during apnea. Thus potential conflict may cause both cardiac changes and arrhythmia development in Lowlanders that travel to higher elevations for work or pleasure. Since high-altitude HVR appears to be predictive of arrhythmia incidence, further evaluation of low-altitude HVR should be performed for predicting susceptibility to high-altitude bradyarrhythmia. The lack of arrhythmias in Sherpa suggests an adaptive mechanism, although it is unclear whether the mechanism behind this response is due to generational adaptation.
GRANTS
Support for this study was partly provided by the Natural Sciences and Engineering Research Council of Canada (RGPIN-2014-06637 to C. D. Steinback and 20150821-01 to P. N. Ainslie), the President’s Grant for the Creative and Performing Arts – Human Performance Scholarship Fund (HPF-162 to C. D. Steinback), and a Canada Research Chair in Cerebrovascular Physiology (950-230970 to P. N. Ainslie).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.A.B., M.S., J.P.M., and C.D.S. conceived and designed research; S.A.B., L.L.S., M.S., J.P.M., and C.D.S. performed experiments; S.A.B., H.D., S.v.D., F.S., and L.R. analyzed data; S.A.B., S.v.D., M.S., J.P.M., and C.D.S. interpreted results of experiments; S.A.B. and C.D.S. prepared figures; S.A.B. drafted manuscript; S.A.B., H.D., S.v.D., L.L.S., F.S., L.R., M.S., P.N.A., C.K.W., R.H., J.P.M., and C.D.S. edited and revised manuscript; S.A.B., H.D., S.v.D., L.L.S., F.S., L.R., M.S., P.N.A., C.K.W., R.H., J.P.M., and C.D.S. approved final version of manuscript.
ACKNOWLEDGMENTS
This study was carried out within the framework of the Ev-K2-CNR Project in collaboration with the Nepal Academy of Science and Technology as foreseen by the Memorandum of Understanding between Nepal and Italy and thanks to contributions from the Italian National Research Council.
REFERENCES
- 1.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kääb S, Couper D, Harris TB, Soliman EZ, Stricker BH, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc 2: e000102, 2013. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anholm JD, Powles AC, Downey R 3rd, Houston CS, Sutton JR, Bonnet MH, Cymerman A. Operation Everest II: arterial oxygen saturation and sleep at extreme simulated altitude. Am Rev Respir Dis 145: 817–826, 1992. doi: 10.1164/ajrccm/145.4_Pt_1.817. [DOI] [PubMed] [Google Scholar]
- 3.Behn C, Dinamarca GA, De Gregorio NF, Lips V, Vivaldi EA, Soza D, Guerra MA, Jiménez RF, Lecannelier EA, Varela H, Silva-Urra JA. Age-related arrhythmogenesis on ascent and descent: “autonomic conflicts” on hypoxia/reoxygenation at high altitude? High Alt Med Biol 15: 356–363, 2014. doi: 10.1089/ham.2013.1092. [DOI] [PubMed] [Google Scholar]
- 4.Boos CJ, Holdsworth DA, Woods DR, O’Hara J, Brooks N, Macconnachie L, Bakker-Dyos J, Paisey J, Mellor A. Assessment of cardiac arrhythmias at extreme high altitude using an implantable cardiac monitor: REVEAL HA Study (REVEAL High Altitude). Circulation 135: 812–814, 2017. doi: 10.1161/CIRCULATIONAHA.116.026584. [DOI] [PubMed] [Google Scholar]
- 5.Burgess KR, Ainslie PN. Central sleep apnea at high altitude. Adv Exp Med Biol 903: 275–283, 2016. doi: 10.1007/978-1-4899-7678-9_19. [DOI] [PubMed] [Google Scholar]
- 6.Cummings P, Lysgaard M. Cardiac arrhythmia at high altitude. West J Med 135: 66–68, 1981. [PMC free article] [PubMed] [Google Scholar]
- 7.Curran LS, Zhuang J, Sun SF, Moore LG. Ventilation and hypoxic ventilatory responsiveness in Chinese-Tibetan residents at 3,658 m. J Appl Physiol (1985) 83: 2098–2104, 1997. doi: 10.1152/jappl.1997.83.6.2098. [DOI] [PubMed] [Google Scholar]
- 8.de Burgh Daly M. Central integration of respiratory and autonomic functions. In: Peripheral Arterial Chemoreceptors and Respiratory-Cardiovascular Integration. Oxford, UK; New York: Clarendon; Oxford Univ. Press, 1997, p. 225. [Google Scholar]
- 9.De Burgh Daly M, Angell-James JE, Elsner R. Role of carotid-body chemoreceptors and their reflex interactions in bradycardia and cardiac arrest. Lancet 313: 764–767, 1979. doi: 10.1016/S0140-6736(79)91218-2. [DOI] [PubMed] [Google Scholar]
- 10.Dempsey JA, Powell FL, Bisgard GE, Blain GM, Poulin MJ, Smith CA. Role of chemoreception in cardiorespiratory acclimatization to, and deacclimatization from, hypoxia. J Appl Physiol (1985) 116: 858–866, 2014. doi: 10.1152/japplphysiol.01126.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duplain H, Vollenweider L, Delabays A, Nicod P, Bärtsch P, Scherrer U. Augmented sympathetic activation during short-term hypoxia and high-altitude exposure in subjects susceptible to high-altitude pulmonary edema. Circulation 99: 1713–1718, 1999. doi: 10.1161/01.CIR.99.13.1713. [DOI] [PubMed] [Google Scholar]
- 12.Hackett PH, Reeves JT, Reeves CD, Grover RF, Rennie D. Control of breathing in Sherpas at low and high altitude. J Appl Physiol Respir Environ Exerc Physiol 49: 374–379, 1980. doi: 10.1152/jappl.1980.49.3.374. [DOI] [PubMed] [Google Scholar]
- 13.Halperin BD, Sun S, Zhuang J, Droma T, Moore LG. ECG observations in Tibetan and Han residents of Lhasa. J Electrocardiol 31: 237–243, 1998. doi: 10.1016/S0022-0736(98)90139-X. [DOI] [PubMed] [Google Scholar]
- 14.Hansen J, Sander M. Sympathetic neural overactivity in healthy humans after prolonged exposure to hypobaric hypoxia. J Physiol 546: 921–929, 2003. doi: 10.1113/jphysiol.2002.031765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinkle DE, Wiersma W, Jurs SG. Multiple-comparison procedures. In: Applied Statistics for the Behavioral Sciences. New York: Houghton Mifflin, 2003, p. 370–390. [Google Scholar]
- 16.Karliner JS, Sarnquist FF, Graber DJ, Peters RM Jr, West JB. The electrocardiogram at extreme altitude: experience on Mt. Everest. Am Heart J 109: 505–513, 1985. doi: 10.1016/0002-8703(85)90555-1. [DOI] [PubMed] [Google Scholar]
- 17.Kollai M, Koizumi K. Reciprocal and non-reciprocal action of the vagal and sympathetic nerves innervating the heart. J Auton Nerv Syst 1: 33–52, 1979. doi: 10.1016/0165-1838(79)90004-3. [DOI] [PubMed] [Google Scholar]
- 18.Lombardi C, Meriggi P, Agostoni P, Faini A, Bilo G, Revera M, Caldara G, Di Rienzo M, Castiglioni P, Maurizio B, Gregorini F, Mancia G, Parati G; HIGHCARE Investigators . High-altitude hypoxia and periodic breathing during sleep: gender-related differences. J Sleep Res 22: 322–330, 2013. doi: 10.1111/jsr.12012. [DOI] [PubMed] [Google Scholar]
- 19.Macefield VG, Wallin BG. Effects of static lung inflation on sympathetic activity in human muscle nerves at rest and during asphyxia. J Auton Nerv Syst 53: 148–156, 1995. doi: 10.1016/0165-1838(94)00174-I. [DOI] [PubMed] [Google Scholar]
- 20.Malconian M, Hultgren H, Nitta M, Anholm J, Houston C, Fails H. The sleep electrocardiogram at extreme altitudes (Operation Everest II). Am J Cardiol 65: 1014–1020, 1990. doi: 10.1016/0002-9149(90)91006-R. [DOI] [PubMed] [Google Scholar]
- 21.Masuyama S, Kohchiyama S, Shinozaki T, Okita S, Kunitomo F, Tojima H, Kimura H, Kuriyama T, Honda Y. Periodic breathing at high altitude and ventilatory responses to O2 and CO2. Jpn J Physiol 39: 523–535, 1989. doi: 10.2170/jjphysiol.39.523. [DOI] [PubMed] [Google Scholar]
- 22.Masuyama S, Shinozaki T, Kohchiyama S, Okita S, Kimura H, Honda Y, Kuriyama T. Heart rate depression during sleep apnea depends on hypoxic chemosensitivity. A study at high altitude. Am Rev Respir Dis 141: 39–42, 1990. doi: 10.1164/ajrccm/141.1.39. [DOI] [PubMed] [Google Scholar]
- 23.Morgan BJ, Crabtree DC, Palta M, Skatrud JB. Combined hypoxia and hypercapnia evokes long-lasting sympathetic activation in humans. J Appl Physiol (1985) 79: 205–213, 1995. doi: 10.1152/jappl.1995.79.1.205. [DOI] [PubMed] [Google Scholar]
- 24.Narkiewicz K, Somers VK. The sympathetic nervous system and obstructive sleep apnea: implications for hypertension. J Hypertens 15: 1613–1619, 1997. doi: 10.1097/00004872-199715120-00062. [DOI] [PubMed] [Google Scholar]
- 25.Paton JF, Boscan P, Pickering AE, Nalivaiko E. The yin and yang of cardiac autonomic control: vago-sympathetic interactions revisited. Brain Res Brain Res Rev 49: 555–565, 2005. doi: 10.1016/j.brainresrev.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Rasmussen PV, Nielsen JB, Skov MW, Pietersen A, Graff C, Lind B, Struijk JJ, Olesen MS, Haunsø S, Køber L, Svendsen JH, Holst AG. Electrocardiographic PR interval duration and cardiovascular risk: results from the Copenhagen ECG study. Can J Cardiol 33: 674–681, 2017. doi: 10.1016/j.cjca.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Reite M, Jackson D, Cahoon RL, Weil JV. Sleep physiology at high altitude. Electroencephalogr Clin Neurophysiol 38: 463–471, 1975. doi: 10.1016/0013-4694(75)90188-1. [DOI] [PubMed] [Google Scholar]
- 28.Seals DR, Suwarno NO, Dempsey JA. Influence of lung volume on sympathetic nerve discharge in normal humans. Circ Res 67: 130–141, 1990. doi: 10.1161/01.RES.67.1.130. [DOI] [PubMed] [Google Scholar]
- 29.Shattock MJ, Tipton MJ. ‘Autonomic conflict’: a different way to die during cold water immersion? J Physiol 590: 3219–3230, 2012. doi: 10.1113/jphysiol.2012.229864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96: 1897–1904, 1995. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinback CD, Breskovic T, Frances M, Dujic Z, Shoemaker JK. Ventilatory restraint of sympathetic activity during chemoreflex stress. Am J Physiol Regul Integr Comp Physiol 299: R1407–R1414, 2010. doi: 10.1152/ajpregu.00432.2010. [DOI] [PubMed] [Google Scholar]
- 32.Steinback CD, Poulin MJ. Ventilatory responses to isocapnic and poikilocapnic hypoxia in humans. Respir Physiol Neurobiol 155: 104–113, 2007. doi: 10.1016/j.resp.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Tipton M. The effect of clothing on “diving bradycardia” in man during submersion in cold water. Eur J Appl Physiol Occup Physiol 59: 360–364, 1989. doi: 10.1007/BF02389811. [DOI] [PubMed] [Google Scholar]
- 34.Woods DR, Allen S, Betts TR, Gardiner D, Montgomery H, Morgan JM, Roberts PR. High altitude arrhythmias. Cardiology 111: 239–246, 2008. doi: 10.1159/000127445. [DOI] [PubMed] [Google Scholar]
- 35.Xie A, Skatrud JB, Puleo DS, Morgan BJ. Exposure to hypoxia produces long-lasting sympathetic activation in humans. J Appl Physiol (1985) 91: 1555–1562, 2001. doi: 10.1152/jappl.2001.91.4.1555. [DOI] [PubMed] [Google Scholar]
- 36.Zhuang J, Droma T, Sun S, Janes C, McCullough RE, McCullough RG, Cymerman A, Huang SY, Reeves JT, Moore LG. Hypoxic ventilatory responsiveness in Tibetan compared with Han residents of 3,658 m. J Appl Physiol (1985) 74: 303–311, 1993. doi: 10.1152/jappl.1993.74.1.303. [DOI] [PubMed] [Google Scholar]