Abstract
Monocytes/macrophages differentiating from bone marrow (BM) cells pulsed for 2 hours at 37°C with a stabilized derivative of prostaglandin E2, 16,16-dimethyl PGE2 (dmPGE2), migrated less efficiently toward a chemoattractant than monocytes/macrophages differentiated from BM cells pulsed with vehicle. To confirm that the effect on BM cells was long lasting and to replicate human BM transplantation, chimeric mice were established with donor BM cells pulsed for 2 hours with dmPGE2 before injection into marrow-ablated congenic recipient mice. After 12 weeks, when high levels (90%) of engraftment were obtained, regenerated BM-derived monocytes/macrophages differentiating in vitro or in vivo migrated inefficiently toward the chemokines colony-stimulating factor-1 (CSF-1) and chemokine (C-C motif) ligand 2 (CCL2) or thioglycollate, respectively. Our results reveal long-lasting changes to progenitor cells of monocytes/macrophages by a 2-hour dmPGE2 pulse that, in turn, limits the migration of their daughter cells to chemoattractants and inflammatory mediators.
Prostaglandin E2 (PGE2), a derivative of arachidonic acid, can regulate immune responsiveness by direct effects on mature immune cells [1]. Myeloid progenitor cells in the bone marrow (BM) are also regulated by a PGE2-rich environment. We have demonstrated reduced immunogenic properties of dendritic cells (DCs) differentiated from progenitors in the BM of mice implanted 3 days prior with subcutaneous pellets releasing PGE2 [2]. The effects were long-lasting because DCs differentiating from the BM of both 16-week-engrafted PGE2-chimeric mice and serial 16- week-engrafted PGE2-chimeric mice had similar sustained, poor immunogenic properties, suggesting epigenetic modification of hematopoietic stem and progenitor cells [2]. This hypothesis was further supported by experimentation with the demethylating agent 5-aza 2-deoxycytidine in UV-chimeric mice [3]. We have described previously experiments with indomethacin showing that UV irradiation of skin modulates DC progenitors indirectly in BM via the intermediate PGE2 [4].
Pulsing of human cord blood donor cells with a stabilized derivative of PGE2, 16,16-dimethyl-PGE2 (dmPGE2) prior to infusion is safe [5,6]. We now report that a similar 2-hour pulse of murine BM cells with dmPGE2 reduced the migration capabilities of monocytes/macrophages differentiating from those BM cells significantly both in vitro and in vivo.
Methods
Pulsing BM cells with dmPGE2
All experiments complied with the ARRIVE guidelines and were performed with the approval of the Telethon Kids Institute Animal Ethics Committee. As shown previously for umbilical cord cell preparations [6], freshly isolated BM cell suspensions (107 cell/mL) [2–4] were pulsed for 2 hours at 37°C with 10 μmol/L dmPGE2 (Sigma-Aldrich, St. Louis, MO). dmPGE2 was obtained dissolved in methyl acetate, which was evaporated off at 4°C in argon, an inert gas, as per the manufacturer’s instructions; the remaining precipitate was then dissolved in RPMI-1640 supplemented with 10% FCS (HyClone, GE Health Care Life Sciences, Logan, UT). As a control, cells were incubated with an equivalent volume of RPMI-1640 with 10% FCS for 2 hours at 37°C.
Generating dmPGE2-chimeric mice
BM cells were isolated from naive congenic B6.SJL-Ptprca mice (CD45.1 alloantigen) and then incubated at 37°C for 2 hours with 10 μmol/L dmPGE2 or control medium, as described above. Eight-week-old C57BL/6J mice (recipients, CD45.2 alloantigen) were gamma-irradiated (2 × 550 rad) using a 137Cs source (Gammacell 3000 Elan, MDS Nordion, Ottawa, Canada) before injection of 2 × 106 BM cells that had been pulsed with dmPGE2 (dmPGE2-chimeric mice) or control medium (control-chimeric mice).
Culture of BM cells for differentiation of macrophages and chemotaxis toward CSF-1 and CCL2
Macrophages were differentiated from BM cells (after a dmPGE2 pulse or after isolation from chimeric mice) as described previously [7–9]. Briefly, BM cells were cultured in RPMI-10 and 0.6 ng/ mL (50 IU/mL) CSF-1 (gift from Dr. E.R. Stanley) for 24 h. Nonadherent cells were then collected and cultured with 12 ng/ mL CSF-1 for 3 days (replated on day 2), followed by 5 days with 120 ng/mL CSF-1 before use. Adherent cells were uplifted using PBS containing 2 mmol/L ethylenediaminetetraacetic acid (EDTA). BM-differentiated macrophages (2.5 × 105) were seeded, in replicate, into Transwell inserts with 8 μm pores (BD Biosciences) in 200 μL of RPMI-10 (CSF-1-free or CCL-2-free) and inserts were placed into a 24-well companion plate with complete medium containing 120 ng/mL CSF-1 or 20 ng/mL CCL2, respectively. Although CSF-1 is necessary for macrophage growth and differentiation, it is also a potent chemokine that stimulates macrophage migration [8,9]. CCL2 is a macrophage chemoattractant with a recognized ability to recruit monocytes to sites of inflammation [10]. The macrophages migrated for 5 hours (37°C, 5% CO2) before fixation of inserts in 4% paraformaldehyde, staining for 5 min with NucBlue-fixed cell stain (Life Technologies, Australia), and imaging by confocal microscopy. Migrated cells were counted for 10 representative fields per insert at 20 × magnification.
Assay of macrophage migration into the peritoneal cavity
The migration capabilities of monocytes/macrophages into the peritoneal cavity of control-chimeric and dmPGE2-chimeric mice were examined by intraperitoneal injection of 1 mL of thioglycollate (3.8% Medium Brewer Modified, Becton Dickinson). After 3 days, the peritoneal cavity was washed out with saline. The harvested cells were counted and identified objectively [11].
Statistical analyses
There were always at least three independent mice or cell populations per group, with differences judged significantly different (p < 0.05) using an unpaired t test.
Results and discussion
Pulsing of freshly isolated BM cells for 2 hours with dmPGE2 did not alter the rate of growth, proliferation, or differentiation of macrophages after 9 days in culture with CSF-1 (Fig. 1A). Because of our protocol of removing adherent (differentiated) cells after the first 24 hours in culture, differentiation of macrophages from progenitor cells was guaranteed [7,8]. All cells at the end of culture were phenotypically similar (>98% CD11b+F4/80+). However, those macrophages differentiated from dmPGE2-pulsed BM cells migrated less efficiently toward CSF-1 than those differentiated from BM cells pulsed with control medium (Fig. 1B). Reduced expression of the CSF-1 receptor (CSF-1R), also known as CD115, was not responsible (Fig. 1C). Macrophages differentiated from the BM of control-chimeric and dmPGE2-chimeric mice were then analyzed to examine the robustness of the dmPGE2 effect. The initial yield and the rate of growth, proliferation, and differentiation of macrophages after 9 days in culture with CSF-1 were not significantly different between BM cells from control-chimeric and dmPGE2-chimeric mice (Fig. 1D). However, reduced migration toward CSF-1 (Fig. 1E) and CCL2 (Fig. 1G) by macrophages differentiated from the BM of dmPGE2-chimeric mice was found consistently. These differences were not due to reduced expression of the chemokine receptors CSF-1R and CCR2 (Figs. 1F and 1H).
Figure 1.
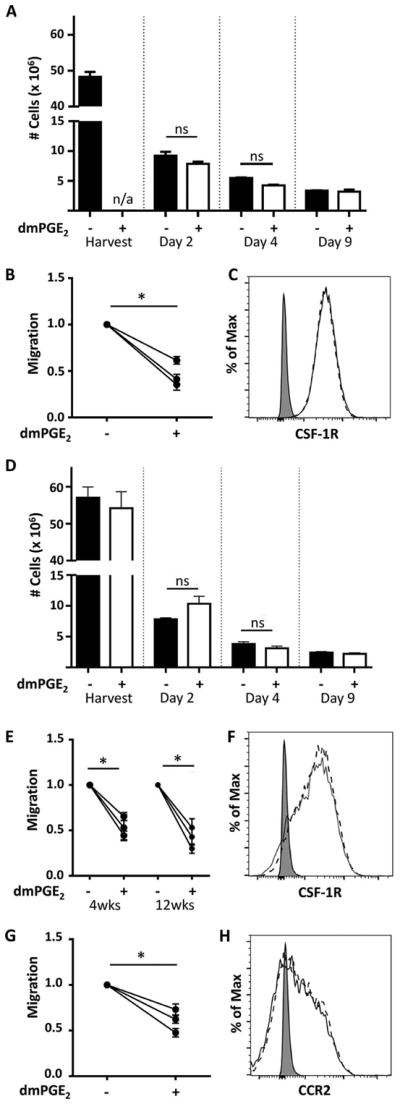
Similar yields from BM cells in culture but reduced migration toward chemoattractants by macrophages differentiated for 9 days from dmPGE2-pulsed BM cells or BM of dmPGE2-chimeric mice. (A) Cell yields from the BM of naive mice and after their culture for 2, 4, and 9 days (control medium-pulsed cells, solid bars; dmPGE2-pulsed cells, open bars). n/a = not applicable. (B) Migration response to CSF-1 (120 ng/mL) by macrophages differentiated from BM cells from three mice pulsed at day 0 for 2 hours with dmPGE2 or control medium. (C) Expression of CSF-1R (CD115) by macrophages differentiated from BM cells pulsed with dmPGE2 (broken line) or control medium (solid line). (D) Cell yields from the BM of control-chimeric and dmPGE2-chimeric mice and after their culture for 2, 4, and 9 days (control-chimeric mice, solid bars; dmPGE2-chimeric mice, open bars). In (A) and (D) (mean ± SEM for three mice/group with cells from each mouse cultured separately), freshly isolated BM cells were seeded at 107 cells/ dish. After 2 days in culture, recovered cells were seeded at 2 × 106/dish and, after 4 days in culture, at 106/dish. ns = not significant. After culture for 2, 4, and 9 days, macrophages were lifted with PBS containing 2 mmol/L EDTA. (E) Migration response to CSF-1 (120 ng/mL) by macrophages differentiated from the BM of control-chimeric and dmPGE2-chimeric mice harvested 4 and 12 weeks after establishment of the chimeric mice. (F) Expression of CSF-1R (CD115) by macrophages differentiated from the BM of 12-week-engrafted dmPGE2-chimeric (broken line) and control-chimeric mice (solid line). (G) Migration response to CCL2 (20 ng/mL) by macrophages differentiated from the BM of control-chimeric and dmPGE2-chimeric mice harvested 12 weeks after establishment of the chimeric mice. (H) As for (F), but expression of CCR2 (CD192) by the same cell types. In (B), (E), and (G), the migration by macrophages differentiated from dmPGE2-pulsed BM cells (B) or BM cells from dmPGE2-chimeric mice (E,G) is expressed as a proportion of the migration by macrophages differentiated from control-medium-pulsed BM cells or BM cells from control-chimeric mice (n = three macrophage preparations/group from three independent mice/group). Each point represents the mean migration (±SEM) by macrophages differentiated from the BM of a single mouse (two filter inserts/mouse; 10 images/insert). Asterisk indicates a significant difference in migration (p < 0.05) between macrophages differentiated from BM cells pulsed with dmPGE2 or control medium (B) or between macrophages differentiated from the BM of control-chimeric and dmPGE2-chimeric mice (E, G). In (C), (F), and (H), the signal from unstained cells (shaded) is shown.
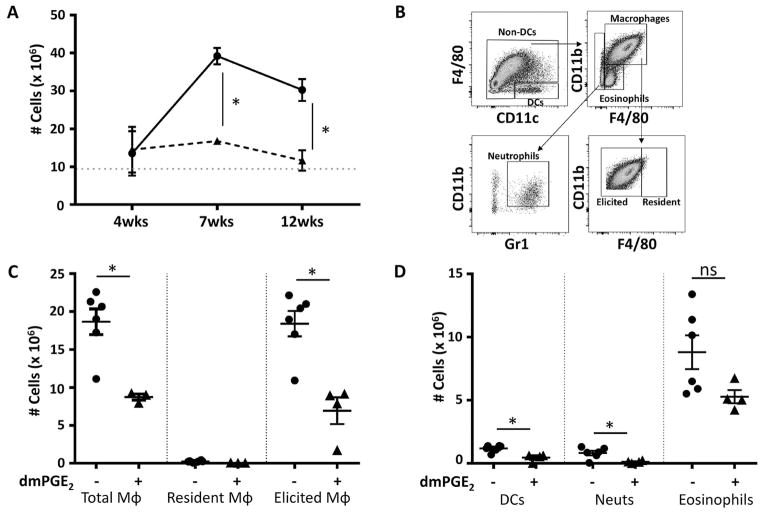
To exclude any artifact due to macrophage differentiation in vitro, accumulation of monocyte-derived macrophages after thioglycollate injection into the peritoneal cavity of control-chimeric and dmPGE2-chimeric mice was examined. For reasons that remain unclear, responses in 4-week chimeric mice were poor, with few macrophages accumulating in the peritoneal cavity. After 7 and 12 weeks of engraftment, the number of cells accumulating in the peritoneal cavity of dmPGE2-chimeric mice was significantly reduced compared with control-chimeric mice (Fig. 2A). There were significantly fewer total macrophages (F4/80+CD11b+), elicited macrophages (F4/80lo/intCD11b+), DCs (F4/80–CD11c+), neutrophils (F4/80–CD11bloGr1+), and eosinophils (F4/80loCD11blo) (Fig. 2B–2D). Expression of the CSF-1R (CD115), CCR2 (CD193), CCR7 (CD197), and MHC II was not different for the elicited macrophages harvested from the peritoneal cavity of the two chimeric mouse types (data not shown).
Figure 2.
Reduced accumulation of macrophages in the peritoneal cavity of dmPGE2-chimeric mice after injection of thioglycollate. Control-chimeric and dmPGE2-chimeric mice were injected intraperitoneally with thioglycollate 4, 7, and 12 weeks after the chimeric mice were established. Cells were harvested from the peritoneal cavity after 3 days. (A) Total cells harvested from the peritoneal cavity of control-chimeric mice (solid line) and dmPGE2-chimeric mice (broken line). The dotted horizontal shows the number of cells harvested from chimeric mice injected with an equal volume of saline. Data are shown as mean ± SEM (n = 6 mice/group). (B) Gating strategy [11] for identification of cell types (C, D) harvested from the peritoneal cavity 3 days after thioglycollate injection of 12-week-engrafted chimeric mice. (C) Number of total, resident, and elicited peritoneal macrophages. (D) Number of DCs, neutrophils (Neuts), and eosinophils. For (C) and (D), data are shown as mean ± SEM (n = 6 mice/group for control-chimeric mice; n = 4 mice/group for dmPGE2-chimeric mice). Asterisk indicates a significant difference (p < 0.05) between cells harvested from control-chimeric and dmPGE2-chimeric mice. ns = not significant.
The actions of PGE2 are both microenvironment and concentration dependent [12]. PGE2 is proinflammatory at early stages of inflammation [1]. It can also play an anti-inflammatory role [13,14] by downregulating systemic inflammation [15] and protecting vascular function [16]. This study suggests a direct and long-lasting effect of dmPGE2 on monocyte/macrophage progenitor cells in the BM. Our study suggests that a 2-hour incubation with dmPGE2 is sufficient to modify BM stem and progenitor cells, resulting in reduced migration capabilities of their monocyte/macrophage progeny.
In patients with leukemia or lymphoma, dmPGE2-pulsed, nonfractionated umbilical cord cells demonstrated increased expression of CXCR4 [5,17], surviving, and cyclin D, and reduced expression of caspase 3 in hematopoietic stem cells [5,6]. In fact, multilineage hematopoiesis has been sustained in patients for 27 months after transplantation with dmPGE2-pulsed umbilical cord cells [18]. In our murine study, dmPGE2-induced increases in the proliferation and differentiation of BM-macrophages were not observed in vitro (Fig. 1A and 1D) and there were no increases in the rate of donor cell engraftment in the BM, spleen, and lymph nodes of the dmPGE2-chimeric mice (Table 1). This was likely because the number of stem and progenitor cells transferred into the gamma-irradiated mice was not rate limiting [19,20]. The outcomes of our study should be further examined to assist patients undergoing hematopoietic stem cell transplantation, particularly because the dmPGE2 pulse is very easy to administer. The safety of such pulsing has been confirmed previously [6]. This study highlights the potential of a short and simple pulse of donor BM cells with dmPGE2 to limit the development of macrophage-driven pathologies. Based on the outcomes of the present study, a dmPGE2 pulse of donor cells should be tested in future experimentation with models of chronic graft-versus-host disease, a condition mediated, at least in part, by CSF-1-dependent donor-derived macrophages [21].
Table 1.
Engraftment of CD45.1+ donor cells into the BM, spleen, and lymph nodes of control-chimeric (C-chim) and dmPGE2-chimeric (dmPGE2-chim) mice
| Weeks reconstituted | BM | Spleen | Lymph nodes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| WT | C-chim | dmPGE2-chim | WT | C-chim | dmPGE2-chim | WT | C-chim | dmPGE2-chim | ||
| Total cells (×106) | 4 | 60.4 ± 6.8 | 46.2 ± 6.2 | 49.6 ± 0.8 | 103.2 ± 4.0 | 64.4 ± 6.0 | 55.4 ± 1.0 | - | - | - |
| 12 | 62.0 ± 11.8 | 50.5 ± 1.6 | 46.7 ± 4.7 | 74.5 ± 6.5 | 70.1 ± 5.4 | 80.4 ± 6.0 | - | - | - | |
| %CD19+B220+ | 12 | 23.1 ± 3.0 | 28.4 ± 1.8 | 27.6 ± 1.4 | 45.5 ± 3.5 | 49.8 ± 1.9 | 50.9 ± 1.7 | 32.7 ± 2.6 | 32.0 ± 2.8 | 30.5 ± 2.9 |
| % CD45.1 | 0.05 ± 0.0 | 97.8 ± 0.6 | 98.4 ± 0.1 | 0.1 ± 0.0 | 99.7 ± 0.1 | 99.7 ± 0.1 | 0.1 ± 0.1 | 99.7 ± 0.1 | 99.6 ± 0.1 | |
| %CD3+CD4+ | 12 | 1.0 ± 0.2 | 1.0 ± 0.1 | 0.7 ± 0.1 | 11.6 ± 1.2 | 13.4 ± 2.0 | 9.9 ± 1.0 | 21.2 ± 4.8 | 33.4 ± 2.3 | 33.3 ± 2.2 |
| % CD45.1 | 0.1 ± 0.0 | 84.9 ± 2.6 | 87.6 ± 3.2 | 0.1 ± 0.0 | 92.1 ± 1.1 | 88.9 ± 1.7 | 0.1 ± 0.0 | 95.1 ± 1.2 | 93.0 ± 0.9 | |
| %CD3+CD4– | 12 | 2.5 ± 0.7 | 2.9 ± 0.1 | 2.1 ± 0.2 | 12.1 ± 0.1 | 8.8 ± 0.8 | 7.4 ± 0.6 | 27.7 ± 6.6 | 29.5 ± 0.3 | 28.1 ± 0.8 |
| % CD45.1 | 0.1 ± 0.0 | 88.7 ± 1.6 | 87.6 ± 0.8 | 0.0 ± 0.0 | 93.7 ± 0.2 | 90.6 ± 1.2 | 0.0 ± 0.0 | 95.7 ± 0.3 | 92.9 ± 0.6 | |
| %F4/80+CD11b+ | 12 | 8.9 ± 1.4 | 13.3 ± 0.9 | 14.5 ± 0.5 | 1.5 ± 0.1 | 2.9 ± 0.6 | 3.0 ± 0.3 | 1.2 ± 0.4 | 1.2 ± 0.5 | 0.6 ± 0.1 |
| % CD45.1 | 1.5 ± 0.4 | 99.8 ± 0.0 | 99.8 ± 0.0 | 4.6 ± 0.6 | 99.1 ± 0.2 | 99.1 ± 0.2 | 3.5 ± 0.7 | 98.9 ± 0.5 | 99.7 ± 0.1 | |
| %CD11c+ | 12 | 2.5 ± 0.2 | 2.3 ± 0.1 | 2.2 ± 0.1 | 3.7 ± 0.0 | 3.7 ± 0.2 | 4.4 ± 0.5 | 3.9 ± 0.5 | 4.8 ± 0.0 | 3.9 ± 0.3 |
| % CD45.1 | 5.5 ± 1.4 | 97.3 ± 0.8 | 98.1 ± 0.4 | 0.4 ± 0.2 | 97.0 ± 0.2 | 96.0 ± 0.0 | 0.4 ± 0.2 | 97.7 ± 0.3 | 96.5 ± 0.5 | |
Comparison of cell yields and cell types from wild-type (WT, nonchimeric) mice are shown at 4 and 12 weeks after injection of cells. Data are shown as mean ± SEM for three individual mice.
Acknowledgments
The authors thank Deborah Strickland for assistance creating the chimeric mice and Bright Blue for their donation of the Nikon C2 confocal microscope.
This work was supported by the Cancer Council Western Australia (PHH, FJP) and the National Health and Medical Research Council, Australia (572660 and 1067209 to PHH).
Footnotes
Conflict of interest
LIZ is a science advisory board member of Fate Therapeutics, Scholar Rock, and Marauder Therapeutics.
References
- 1.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott NM, Ng RLX, Gorman S, Norval M, Waithman J, Hart PH. Prostaglandin E2 imprints a long-lasting effect on DC progenitors in the bone marrow. J Leuk Biol. 2014;95:225–232. doi: 10.1189/jlb.0513294. [DOI] [PubMed] [Google Scholar]
- 3.Ng RLX, Scott NM, Strickland DH, et al. Altered immunity and dendritic cell activity in the periphery of mice after long-term engraftment with bone marrow from UV-irradiated mice. J Immunol. 2013;190:5471–5484. doi: 10.4049/jimmunol.1202786. [DOI] [PubMed] [Google Scholar]
- 4.Ng RLX, Bisley JL, Gorman S, Norval M, Hart PH. UV-irradiation of mice reduces the competency of bone marrow-derived CD11c+ cells via an indomethacin-inhibitable pathway. J Immunol. 2010;185:7207–7215. doi: 10.4049/jimmunol.1001693. [DOI] [PubMed] [Google Scholar]
- 5.Hagedorn EJ, Durand EM, Fast EM, Zon LI. Getting more for your marrow: boosting hematopoietic stem cell numbers with PGE2. Exp Cell Res. 2014;329:220–226. doi: 10.1016/j.yexcr.2014.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cutler C, Multani P, Robbins D, et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122:3074–3081. doi: 10.1182/blood-2013-05-503177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanley ER. Murine bone marrow-derived macrophages. Methods Mol Biol. 1990;5:299–302. doi: 10.1385/0-89603-150-0:299. [DOI] [PubMed] [Google Scholar]
- 8.Pixley FJ. Macrophage migration and its regulation by CSF-1. Int J Cell Biol. 2012;2012:501962. doi: 10.1155/2012/501962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dwyer AR, Mouchemore KA, Steer JH, et al. Src family kinase expression and subcellular localization in macrophages: implications for their role in CSF-1-induced macrophage migration. J Leuk Biol. 2016;100:163–175. doi: 10.1189/jlb.2A0815-344RR. [DOI] [PubMed] [Google Scholar]
- 10.Deshmane SL, Kremley S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interfer Cyt Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosn EE, Cassado AA, Govoni GR, et al. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci USA. 2010;107:2568–2573. doi: 10.1073/pnas.0915000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osma-Garcia IC, Punzon C, Fresno M, Diaz-Munoz MD. Dose-dependent effects of prostaglandin E2 in macrophage adhesion and migration. Eur J Immunol. 2016;46:677–688. doi: 10.1002/eji.201545629. [DOI] [PubMed] [Google Scholar]
- 13.Rieser C, Bock G, Klocher H, Bartsch G, Thurnher M. Prostaglandin E2 and tumor necrosis factor a cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603–1608. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agard M, Asakrah S, Morici LA. PGE2 suppression of innate immunity during mucosal bacterial infection. Front Cell Infect Microbiol. 2013;3:45. doi: 10.3389/fcimb.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffin R, O’Connor RA, Crittenden S, et al. Prostaglandin E2 constrains systemic inflammation through an innate lymphoid cell-IL-22 axis. Science. 2016;351:1333–1338. doi: 10.1126/science.aad9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi J, Johansson J, Woodling NS, Wang Q, Montine TJ, Andreasson K. The prostaglandin E2 E-prostanoid 4 receptor exerts anti-inflammatory effects in brain innate immunity. J Immunol. 2010;184:7207–7218. doi: 10.4049/jimmunol.0903487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Kim HT, Nellore A, et al. Prostaglandin E2 promotes survival of naïve UCB T cells via the Wnt/β-catenin pathway and alters immune reconstitution. Blood Cancer J. 2014;4:e178. doi: 10.1038/bcj.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lord AM, North TE, Zon LI. Prostaglandin E2: making more of your marrow. Cell Cycle. 2007;6:3054–3057. doi: 10.4161/cc.6.24.5129. [DOI] [PubMed] [Google Scholar]
- 19.Porter RL, Georger M, Bromberg O, et al. Prostaglandin E2 increases hematopoietic stem cell survival and accelerates hematopoietic recovery after radiation injury. Stem Cells. 2013;31:372–383. doi: 10.1002/stem.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frisch BJ, Porter RL, Gigliotti BJ, et al. In vivo prostaglandin E2 treatment alters the bone marrow microenvironment and preferentially expands short-term hematopoietic stem cells. Blood. 2009;114:4054–4063. doi: 10.1182/blood-2009-03-205823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexander KA, Flynn R, Lineburg KE, et al. CSF-1-dependent donor-derived macrophages mediate chronic graft-versus-host disease. J Clin Invest. 2014;124:4266–4280. doi: 10.1172/JCI75935. [DOI] [PMC free article] [PubMed] [Google Scholar]