Abstract
The c-MYB transcription factor is a key regulator of hematopoietic cell proliferation and differentiation, and dysregulation of c-MYB activity often associates with various hematological disorders. Yet, its pathogenic role remains largely unknown due to lack of suitable animal models. Here, we report a detail characterization of a c-myb-gfp transgenic zebrafish harboring c-Myb hyperactivity (named c-mybhyper). This line exhibits abnormal granulocyte expansion that resembles human myelodysplastic syndrome (MDS) from embryonic stage to adulthood. Strikingly, a small portion of c-mybhyper adult fish develops acute myeloid leukemia-like or acute lymphoid leukemia-like disorders with age. The myeloid and lymphoid malignancies in c-mybhyper adult fish are likely caused by the hyperactivity of c-myb, resulting in the dysregulation of a number of cell-cycle-related genes and hyperproliferation of hematopoietic precursor cells. Finally, treatment with c-myb target drug flavopiridol can relieve the MDS-like symptoms in both c-mybhyper embryos and adult fish. Our study establishes a zebrafish model for studying the cellular and molecular mechanisms underlying c-Myb-associated leukemogenesis as well as for anti-leukemic drug screening.
INTRODUCTION
The proto-oncogene c-myb is a master regulator essential for proliferation and differentiation of hematopoietic cells during normal hematopoiesis.1,2 Studies of c-MYB-deficient mice and zebrafish have revealed that c-MYB plays essential roles in various stages of hematopoiesis, including hematopoietic stem cell (HSC) development,3 erythropoiesis,4 myelopoiesis5 and lymphopoiesis.6,7 The importance of c-MYB is exemplified by its involvement in hematological diseases in animals and humans. Aberrant activation or ectopic expression of c-MYB in vitro has been shown to contribute to the transformation of hematopoietic progenitors into leukemic cells.8 In avian and in mice, c-MYB truncated by virus targeting often leads to its aberrant activity, and causes leukemia transformation.9,10 Conversely, suppression of c-MYB activity in mice inhibits aggressive acute myelogenous leukemia (AML).11 Clinically, c-MYB has been found to be highly expressed in leukemic cells in patients with AML, chronic myeloid leukemia and acute lymphoblastic leukemia (ALL), and it is essential for the proliferation and maintenance of leukemic cells.2,12,13 Several recent reports have identified genetic lesions, including chromosomal translocation,14 genomic duplication15 and mutations,16 that alter the c-MYB activity in human lymphoid and myeloid leukemias. Collectively, these studies have documented that c-MYB plays a key role in human leukemogenesis. However, the molecular basis underlying c-MYB-associated leukemogenesis remains undefined and effective treatment for leukemia patients with aberrant c-MYB activity is still lacking.
Zebrafish, which share high similarities in blood contents and genetic regulatory networks to mammals,17 have emerged as an excellent model organism for studying the pathogenesis of some hematological disorders18 as well as for drug discovery.19 Despite increasing numbers of zebrafish models with hematologic malignancies have been generated by mutagenesis or by overexpressing key oncogenic proteins in the past several years,20,21 a zebrafish malignant model associated with c-MYB hyperactivity remains unavailable.
Here, we investigated the induction of myeloid and lymphoid leukemia in transgenic zebrafish with c-myb hyperactivity. We found that a c-myb-gfp transgenic zebrafish line generated previously22 overexpresses WT c-Myb and a hyperactive fusion c-Myb because of the duplication of the c-myb locus. The c-myb-overexpressing zebrafish, referred as to c-mybhyper (c-myb with hyperactivity), display hematopoietic perturbation at the embryonic stages and can develop myeloid and lymphoid leukemia-like phenotypes in adulthood. Our findings show that c-Myb functions as a driver for leukemogenesis in vivo by promoting hematopoietic cell proliferation and the c-mybhyper zebrafish may serve as a suitable animal model for anti-leukemia drug screening.
MATERIALS AND METHODS
Zebrafish husbandry
All experiments involving zebrafish were performed in accordance with the guidelines laid down by the Institutional Animal Care and Use Committee of Southern Medical University. Zebrafish (3 days–24 months old) of either sex were maintained as described previously.23,24 The following strains were used: AB, c-myb-gfp,22 c-mybhkz3 (a loss-of-function c-myb mutant),25 rag2-dsRed26 and lyz-dsRed.27
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed with antisense digoxigenin-labeled RNA probes, according to standard protocols.28
Sudan black staining
Fixed embryos were incubated in Sudan black B (Sigma-Aldrich, St Louis, MO, USA; 199664) solution and washed as described previously.29
Leukemia transplantation
Leukemic cell suspensions were prepared from c-mybhyper/lyz-dsRed (myelodys-plastic syndrome (MDS)-like), c-mybhyper (AML-like) or c-mybhyper/rag2-dsRed (ALL-like) fish as previously described.20 Two days after receiving a sublethal dose of radiation (25 Gy), 0.2 million cells were injected intracardially into the irradiated recipients using a glass capillary needle (World Precision Instruments, Sarasota, FL, USA; 1B100-6).
Bromodeoxyuridine labeling
Cytological analysis
Peripheral blood (PB) and kidney marrow (KM) were re-suspended in ice-cold phosphate-buffered saline with 5% fetal bovine serum, followed by cytospins at 400 r.p.m. for 3 min. The cells were then stained with Giemsa (Merck, Darmstadt, Germany; 1.09204.0500) and May-Grunwald’s eosin methylene blue (Merck; 1.01424.0500) according to the manufacturer’s instructions. Blood cells of KM and PB were calculated manually based on their morphologies.30
Treatment with chemotherapeutic agents
Embryos were soaked in egg water containing cytarabine (Pfizer, Milano, Italy), quizartinib (LC Laboratories, Woburn, MA, USA) or flavopiridol (Santa Cruz, Dallas, TX, USA; sc-202157) for drug treatment. Adult fish were intraperitoneally injected with cytarabine (600–2000 mg/kg) and flavopiridol (30–130 mg/kg) once daily for 4 days. The doses for intraperitoneal injection were based on the trials in murine models,31,32 and 4–20-fold higher doses were applied in adult fish.
Statistical analysis
The differences between categorical variables were analyzed by Fisher’s exact tests. Continuous variables were compared by two-tailed Student’s t-tests. In all graphs, error bars reflect mean ± s.d. A P-value less than 0.05 was considered significant. P-values were not adjusted for multiple comparisons and the variation was similar between the groups that were compared. No statistical methods were used to predetermine sample size. The sample size was determined based on numbers reported in previous studies and no samples were excluded. Experiments were not randomized, and investigators were not blinded to allocation during experiments and outcome assessment.
RESULTS
Two different c-myb transcripts, a wild-type and a truncated form, are produced from the c-myb PAC in the c-myb-gfp transgenic line
North et al.22 have described the generation of a c-myb-gfp transgenic zebrafish line for labeling c-myb-positive hematopoietic stem cells. Unexpectedly, when examined the phenotype of c-mybhkz3−/−; c-myb-gfp+/− embryos, we found that the c-mybhkz3−/− mutant phenotypes were completely rescued (data not shown). This result prompted us to speculate that a functional c-Myb must be produced in the c-myb-gfp transgenic line. Consistent with this idea, whole-mount in situ hybridization staining showed that c-myb expression in the aorta-gonad-mesoderm, caudal hematopoietic tissue and kidney was significantly increased in c-myb-gfp transgenic line compared with that in WT control fish (Figure 1a), suggesting c-myb might be overproduced in this line. To prove this is indeed the case, we sequenced the c-myb PAC in c-myb-gfp transgenic fish and found two duplicated sequences in the modified c-myb PAC: a 485-bp sequence (including a 315-bp core promoter region and a 170-bp 5′ UTR of c-myb) inserted before the translation start site (Figure 1b and Supplementary Figure S1A); and a large region from pWSMK-T vector to c-myb intron 10 inserted into the breakpoint within intron 10 (Figure 1b and Supplementary Figure S1B). 5′ and 3′-rapid amplification of cDNA ends analysis revealed that two different c-myb transcripts were produced from the c-myb PAC in the c-myb-gfp transgenic fish (Figure 1c and Supplementary Figure S1C). One transcript c-myb-WT was produced from the second c-myb core promoter and was identical to the endogenous c-myb (Figure 1c). The other transcript c-myb-T1 (4.3 kb), generated from the first c-myb core promoter, comprised of a truncated c-myb (from exon 1 to exon 10) followed by a near full-length c-myb (from exon 2 to exon 15) (Figure 1c). The c-myb-T1 transcript is predicted to produce a large fusion protein, in which a truncated c-Myb lacking the majority of negative-regulatory region33 is fused with a nearly full-length c-Myb (lacking the first 8 aa) (Figure 1c and Supplementary Figure S1D). Quantification analysis indicated that the ratio of endogenous c-myb, transgenic c-myb-WT and c-myb-T1 in the KM of adult c-myb-gfp transgenic fish was 1:5:2 (Figure 1d), confirming that c-myb transcripts are indeed overproduced in the c-myb-gfp transgenic line. As c-Myb-T1 fusion protein contains a truncated c-Myb lacking the inhibitory domain, we reasoned that the c-Myb-T1 had a higher transactivation activity as previously reported in mammals.33 Indeed, luciferase reporter assay confirmed that c-Myb-T1 displayed a higher transactivation activity in activating the lect2l gene promoter (the zebrafish ortholog of the chicken c-Myb target gene min-134) than c-Myb-WT (Supplementary Figure S1E). Thus, the c-myb-gfp transgenic fish appears to produce higher level of c-Myb-WT and a hyperactive truncated c-Myb-T1 and it is therefore referred as to c-mybhyper.
Figure 1.
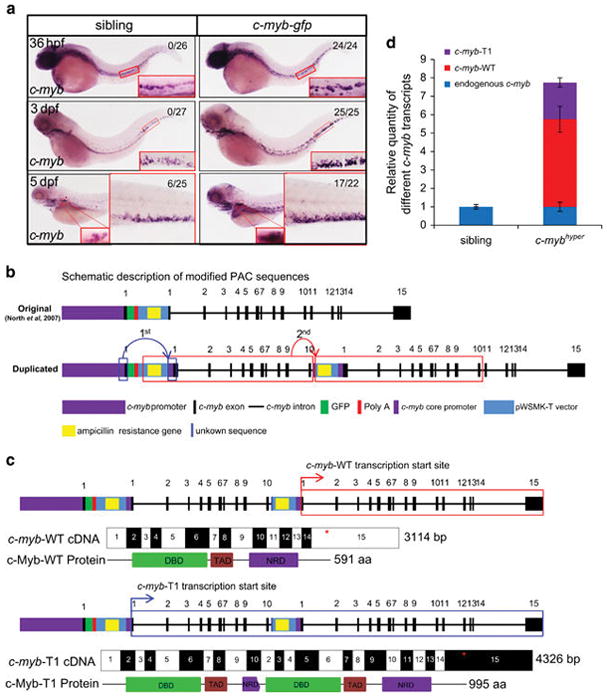
c-myb-WT and c-myb-T1 transcripts were produced from the c-myb PAC in c-myb-gfp. (a) c-myb mRNA is overproduced in c-myb-gfp transgenic zebrafish. Whole-mount in situ hybridization of c-myb expression at 36 hpf, 3 dpf and 5 dpf. Enlarged details of the aorta-gonad-mesoderm, caudal hematopoietic tissue (CHT) and kidney are boxed in red (×20). Numbers in each panel indicate the number of embryos with elevated expression of markers out of total number of fish (Fisher’s exact tests, P<0.01). (b) Two duplications were found in the modified PAC in c-myb-gfp transgenic zebrafish. The first repeat (1st, 485-bp repetitive mini promoter) is indicated by blue box, and the second repeat (2nd, pWSMK-T to c-myb intron 10 region) is indicated by red box. The inserting sites of repetitive sequence are indicated by arrows. Exons of c-myb (black bar); pWSMK-T (light blue bar) including GFP (green bar), SV40 polyA signal (red bar) and the reversed ampicillin resistance gene (AmpR, yellow bar); 77-bp additional unknown sequence is indicated (blue bar). (c) Transcripts and proteins of c-myb-WT and c-myb-T1. The transcription start sites of c-myb-WT and c-myb-T1 are indicated by red arrows (upper panels) and blue arrows (lower panels), respectively. Stop codons are marked by asterisk. The DNA-binding domain (DBD), transactivation domain (TAD) and negative-regulatory domain (NRD) of c-Myb protein are indicated. (d) Relative expression of different c-myb transcripts in 1-year adult kidney marrow (sibling and c-mybhyper, n =15 and n =12, respectively).
Aberrant c-myb hyperactivity results in accumulation of abnormal granulocytes in early development
It has been shown that the appropriate levels of c-Myb are important for regulating the distinct differentiation steps during hematopoietic cell development,35 and aberrant c-MYB activities have been known to associate with human myeloid leukemia.36,37 We therefore explored the effects of aberrant c-Myb production on myeloid cell development in c-mybhyper embryos and larvae using lineage-specific markers. We found that early myeloid markers, c/epba (CCAAT/enhancer-binding protein a)38 and lcp (lymphocyte cytosolic protein 1),39 were increased in c-mybhyper embryos (Supplementary Figures S2A and B). Likewise, the expression of granulocytic markers, lyz (lysozyme C)40 and mpx (myeloid-specific peroxidase),41 was also elevated (Figure 2a and Supplementary Figure S2C). In contrast, the macrophage lineage markers, mfap4 (microfibrillar-associated protein 4)42 and c-fms (colony-stimulating factor-1 receptor),43 did not show obvious increase in c-mybhyper embryos (Supplementary Figures S2D and E). These data suggest a robust expansion of granulocytic lineage in c-mybhyper embryos. This was further supported by the increased Sudan black (SB) staining, which preferentially marks granulocytes in zebrafish,44 in the caudal hematopoietic tissue and KM in c-mybhyper larvae (Figures 2b–d). Notably, the SB+ granulocytes in c-mybhyper appeared to be larger in size and darker in SB staining (Figures 2b and c), compared with their sibling controls, indicating that the increased granulocytic cells perhaps as a result of early developmental defect.
Figure 2.
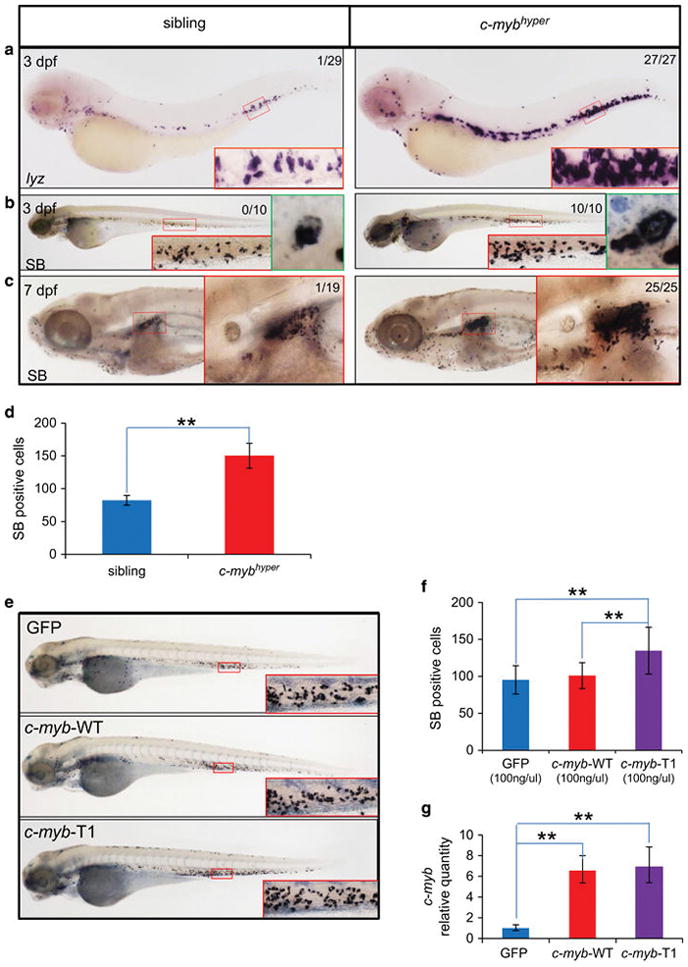
Abnormal myeloid cell expansion in c-myb-gfp embryos and larvae. (a–d) Accumulation of neutrophils in c-myb-gfp embryos and larvae. Whole-mount in situ hybridization of lyz (a) expression at 3 dpf in c-myb-gfp fish and non-transgenic siblings. SB staining in 3 dpf (b) and 7 dpf (c) larvae. The caudal hematopoietic tissue (CHT) and kidney regions were enlarged in red box (×20). Green boxes (b) show further enlarged region focusing on a single cell (×100). Numbers in each panel indicate the number of embryos with elevated expression of markers out of total number of fish (Fisher’s exact tests, P<0.01). SB+ cell counts in 3 dpf (d) (t-test, n =10; mean ±s.d.; *P<0.05, **P<0.01). (e–g) Ectopically expressed c-myb-WT and c-myb-T1 under the c-myb mini promoter. cDNAs of c-myb-WT-gfp, c-myb-T1-gfp and gfp control driven by c-myb mini promoter were injected into one-cell stage WT embryos, respectively. Representative pictures of SB-positive cell counts in 3 dpf embryos (e). SB+ myeloid cell counts (f) and c-myb transcript levels (g) after injection were calculated and compared at 3 dpf (t-test; gfp, c-myb-WT-gfp, c-myb-T1-gfp, n =21, n =24 and n =23, respectively; mean ±s.d.; *P<0.05, **P<0.01).
To determine the myeloid cell expansion is attributed to single transcripts generated from the PAC or caused by combination effects of both products, we compared the granulocytic lineage expansion ability of the two forms by ectopically expressing c-myb-WT and c-myb-T1 driven by the same promoter in WT embryos. Consistent with the observation that c-Myb-T1 is hyperactive, we found that under the control of the c-myb mini promoter (Supplementary Figure S1A), ectopically expressing c-myb-T1 caused a modest increase of SB+ granulocytes, while c-myb-WT failed to do so (Figures 2e and f), though the expression of both transcripts is comparable (Figure 2g). However, overexpressing either c-myb-WT or c-myb-T1 under a stronger ubiquitous promoter (elongation factor-1 alpha promoter, ef1a) was sufficient to induce granulocytic lineage expansion as indicated by SB staining, though the induction by c-myb-T1 was more profound (Supplementary Figures S2F–H). These data indicate that the myeloid accumulation in c-mybhyper fish is likely to be attributed to additive effects of c-myb-WT and c-myb-T1.
c-mybhyper adult fish display abnormal myeloid cell expansion resembled MDS-like phenotypes mainly caused by proliferation perturbation
MYB hyperactivity has been shown to be main genetic mutations in several types of human lymphoid and myeloid leukemias.2,14,15 To examine the possible development of leukemia-like hematological disorders in c-mybhyper adult fish, KM and PB cells were randomly collected from 3-month-old and 1-year-old c-mybhyper fish or siblings and subjected to cytological and blood cell count analyses. No significant alterations were observed in PB constituents in c-mybhyper fish at 3 months or 1 year (Supplementary Figures S3A and B and Figures 3a and b). However, analysis of KM cells indicates a significant expansion of the myeloid cell population in 3-month-old c-mybhyper fish, along with a corresponding reduction in hematopoietic precursors (Supplementary Figures S3C and D). By 1-year old, the myeloid population had almost doubled, accompanied by obvious reductions in other blood constituents in c-mybhyper fish KM (Figures 3c and d), consistent with myeloid hyperplasia. In accordance with the cytological results, flow cytometry analysis of KM also showed a twofold increase in the myeloid cell population in 1-year-old adult c-mybhyper fish (Supplementary Figures S3E and F). These results indicate marked myelodysplasia in c-mybhyper fish characterized by elevated cell numbers, enlarged cell size and increased cell granularity, resembled the symptoms of human MDS, composed by a heterogeneous group of clonal hematological disorders that are usually diagnosed based on findings in PB, and especially the bone marrow and characterized by ineffective hematopoiesis, showing dysplastic feature in at least one lineage in the bone marrow.45 To further determine if their internal organs were affected, c-mybhyper and control fish were dissected and observed under the microscope. The kidneys in 1-year-old c-mybhyper zebrafish appeared swollen, with an enlarged kidney area and almost fourfold increase in kidney weight compared with siblings (Figures 3e–g). Livers in c-mybhyper fish were also heavier, with enrichment of invaded SB+ granulocytes compared with controls (Figures 3h and i). These defects resembled the effects on organs and invasion observed in myelogenous hematological malignancies. Overall, the blood phenotypes and organ infiltrations in c-mybhyper fish resembled the symptoms of MDS.
Figure 3.
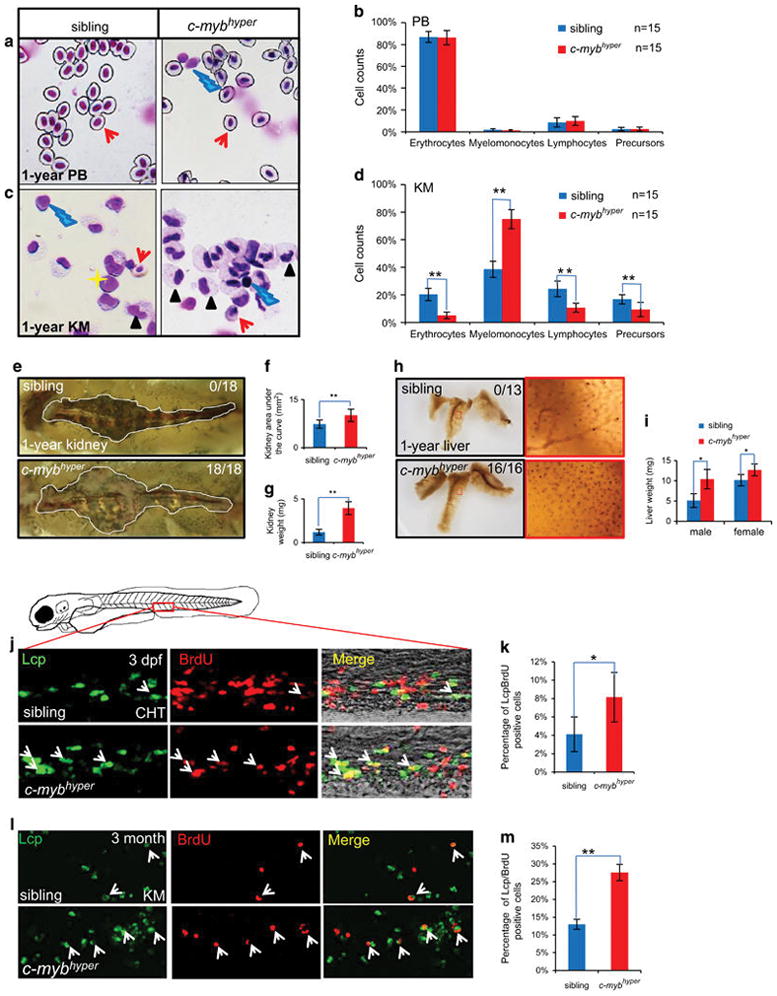
c-mybhyper adult fish exhibit MDS-like phenotypes with abnormal myeloid cell expansion in the kidney. (a–d) Peripheral blood (PB) cells (a) and whole kidney marrow (KM) blood cells (c) in 1-year-old c-mybhyper and sibling fish stained with May-Grunwald/Giemsa. Blood cell counts of PB (b) and KM (d) in siblings (blue bars) and c-mybhyper fish (red bars) were calculated manually based on their morphology. The black asterisks indicate statistical difference (t-test) (n = 15; mean ±s.d.; *P<0.05, **P<0.01). Red arrows, yellow asterisks, black arrowheads and blue lightnings indicate erythrocytes, precursors, myelomonocytes and lymphocytes, respectively. (e–i) Nephromegaly and hepatomegaly occurred in c-mybhyper fish. Kidneys from 1-year transgenic fish were enlarged (e) to 1.4-fold in area (f) and 3.3-fold in weight (g) compared with siblings. (h) SB staining in livers from 1-year transgenic fish showed more SB+ cells than that of siblings. The right red columns show enlarged details of the left red-boxed region. (i) Liver weights in c-mybhyper fish were increased compared with siblings (t-test, sibling and c-mybhyper, n =10 and n =12, respectively; mean ±s.d.; *P<0.05, **P<0.01). Numbers in each panel indicate the number of liver with elevated SB-positive cells out of total number of liver (Fisher’s exact tests, P<0.01) (j–m) Aberrant c-myb activity caused increased proliferation of myeloid cells in embryos (j, k) and KM of adults (l, m). Double staining of bromodeoxyuridine (BrdU)/Lcp (j, l) show BrdU incorporation of caudal hematopoietic tissue (CHT)/KM Lcp+ cells in 3 dpf/3-month c-mybhyper and siblings. Arrows indicate Lcp/BrdU double-positive cells. Percentage of the CHT and KM localized Lcp+ myeloid cells that incorporate BrdU (k, m) in Lcp+ myeloid cells (t-test, sibling and c-mybhyper, n =15 and n =13, respectively; mean ±s.d.; *P<0.05, **P<0.01).
The myeloid cell accumulation in c-mybhyper fish from embryonic stage to adult could be caused by accelerated proliferation or reduced apoptosis. To clarify the cellular mechanisms responsible for myeloid cell expansion in c-mybhyper fish, we monitored cell proliferation and cell death in neutrophils by bromodeoxyuridine incorporation and terminal dexynucleotidyl transferase (TdT)-mediated dUTP nick end labeling assay, respectively. Myeloid cell apoptosis in c-mybhyper embryos and adult KM was comparable to those in sibling control (Supplementary Figures S3G–J), suggesting that the expansion of myeloid cells in c-mybhyper is not caused by reduced apoptosis. On the other hand, myeloid cell bromodeoxyuridine incorporation was significantly increased in c-mybhyper embryos and adult KM (Figures S3j–m), indicating that the expansion of myeloid cells in c-mybhyper fish is the result of increased proliferation.
To further unveil how c-myb hyperactivity caused proliferation perturbation, we examined the expression of 12 cell-cycle-related genes including ccna1,46 ccnb1,47 ccne,47 cdk1,48 pcna49 and cdkn1c,50 which are known to be or predicted to be the direct targets of c-myb. As shown in Table 1, the expression of the cell-cycle-promoting genes ccna1, ccnb1, ccnd1, ccne, ccnh, cdk1, cdk2, cdk7, cdc20 and pcna were upregulated in c-mybhyper MDS KM cells, while the cell-cycle inhibition genes cdkn1c and cdkn2d were downregulated in c-mybhyper MDS KM cells (Table 1). Collectively, these results indicate that c-myb hyperactivity affects the expression of a cluster of cell-cycle-related genes, resulting in myeloid cell proliferation perturbation.
Table 1.
Relative expression of 12 cell-cycle-related genes in sibling and c-mybhyper MDS-like fish
| Gene | Sibling | c-mybhyper |
|---|---|---|
| ccna1 | 1 ±0.10 | 22.34 ±6.63** |
| ccnb1 | 1 ±0.11 | 26.25 ±3.94** |
| ccne | 1 ±0.10 | 12.81 ±1.13** |
| ccnd1 | 1 ±0.13 | 14.01 ±1.71** |
| ccnh | 1 ±0.25 | 264.88 ±58.91** |
| cdk1 | 1 ±0.24 | 7.42 ±1.70** |
| cdk2 | 1 ±0.29 | 39.83 ±14.51** |
| cdk7 | 1 ±0.25 | 239.9 ±54.34** |
| cdc20 | 1 ±0.25 | 73.69 ±16.65** |
| pcna | 1 ±0.24 | 30.00 ±6.65** |
| cdkn1c | 1 ±0.05 | 0.20 ±0.02** |
| cdkn2d | 1 ±0.06 | 0.27 ±0.06** |
The total RNA was collected from four KMs of sibling or c-mybhypereach time, and the experiments were triplicated, totally. T-test, sibling and c-mybhyper, n =12 and n =10, respectively; mean ±s.e.m.;
P<0.01.
c-mybhyper MDS fish can progress to AML and ALL
MDS is the most common hematologic malignancy in the elderly, and approximately one-third of MDS patients will eventually progress to acute leukemia.51 We were therefore keen to test if adult c-mybhyper fish developed AML or ALL (with myeloblasts or lymphoblasts increased) by analyzing blood cell counts or FACS of a large number of 10- 24-month-old adult c-mybhyper fish and siblings. As expected, acute leukemia were never found in 324 of 10–24 months sibling fish. However, 18 AML and 18 ALL were found among 852 of 10- 24-month-old c-mybhyper fish (Table 2).
Table 2.
Hemogram and classification of c-mybhyper fish at 10–24 months
| Classification | Group | Number | Percentages in PB
|
Percentages in KM
|
Leukemic cell types | ||||
|---|---|---|---|---|---|---|---|---|---|
| Myelomonocytes | Lymphocytes | Precursors | Myelomonocytes | Lymphocytes | Precursors | ||||
| Sibling | 14 | 18.5 ±2.6 | 68.0 ±5.3 | 14 ±3.9 | 45.0 ±2.7 | 32.8 ±2.0 | 22.3 ±1.7 | ||
| c-mybhyper-MDS | 47 | 14.8 ±2.4 | 76.3 ±2.4 | 8.9 ±0.8 | 73.1 ±1.9 | 16.5 ±1.4 | 10.5 ±0.9 | Neutrophil | |
| c-mybhyper-AML | I | 2 | 8.7 ±4.2 | 32.8 ±15.9 | 58.5 ±20.0a | 23.0 ±13.5 | 34.5 ±8.5 | 42.0 ±4.9a | Neutrophil |
| II | 12 | 12.3 ±4.6 | 26.1 ±5.1 | 61.6 ±8.0a | 77.8 ±2.8 | 12.5 ±2.3 | 9.7 ±1.1 | Neutrophil | |
| III | 4 | 15.5 ±1.5 | 74.2 ±1.4 | 10.3 ±2.8 | 36.7 ±1.5 | 22.2 ±1.0 | 41.1 ±0.6a | Neutrophil | |
| c-mybhyper-ALL | I | 13 | 6.0 ±2.1 | 89.2 ±3.0b | 4.9 ±1.7 | 27.7 ±5.5 | 60.6 ±6.6b | 11.5 ±4.3 | T-cell |
| II | 5 | 0.9 ±0.2 | 18.8 ±7.1 | 80.3 ±7.2c | 29.3 ±10.3 | 64.4 ±11.1b | 6.8 ±2.4 | B-cell | |
The percentages were indicated by mean ±s.e.m. Adult c-mybhyper fish characterized by >25% precursors with myeloblasts morphology in PB or >40% in KM were divided into AML; and fish characterized by >80% lymphocytes or >60% precursors with lymphoblasts morphology in the PB, and >40% in the KM were divided into ALL. AML-Group I: precursors increased in both PB and KM. AML-Group II: precursors increased in PB. AML-Group III: precursors increased in KM. ALL-Group I: lymphocytes increased in both PB and KM. ALL-Group II: precursors increased in PB and lymphocytes increased in KM (mean ± s.e.m.).
Indicates precursors in PB or KM increased >25% or 40%, respectively, in AML.
Indicates lymphocytes in PB or KM increased >80% or >40%, respectively, in ALL.
Indicates precursors in PB increased >60% in ALL.
The AML-like c-mybhyper fish showed >25% increase in precursors with myeloblasts morphology in the PB or >40% increase of precursors in KM (Figure 4a and Table 2), and some of them showed pathologic external features, including abnormal body contour, exophthalmos, bleeding and abdominal mass (Supplementary Figures S4C–F). Moreover, examination of tissue sections of AML-like c-mybhyper fish showed obvious infiltrations of leukemic cells with myeloblast morphology in many non-hematopoietic tissues including liver, muscles and gills (Figure 4b and Supplementary Figure S5A). The typical granulocytic morphology of the myeloid cells accumulated in the KM (Figure 4a) and infiltrated in non-hematopoietic tissues (Figure 4b and Supplementary Figure S5A) indicates that c-mybhyper fish could progress to AML with granulocytic origin.
Figure 4.
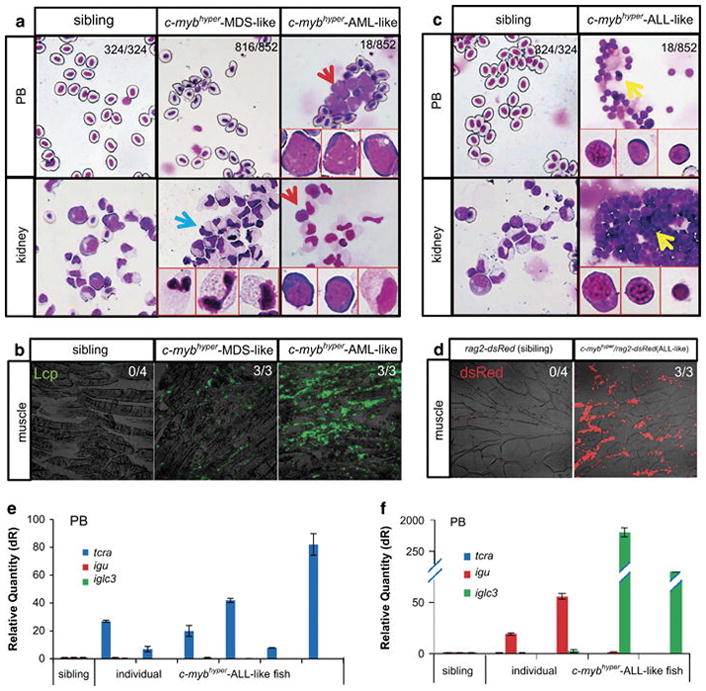
c-mybhyper zebrafish develop AML and ALL in adulthood. (a, b) c-mybhyper zebrafish develop AML in adulthood. (a) May-Grunwald/Giemsa staining of PB cells (upper panels) and KM blood cells (lower panels) were obtained from siblings (left panels) and c-mybhyper zebrafish (middle and right panels). Blue arrows indicate accumulated neutrophils in MDS-like fish KM. Red arrows indicate myeloid blasts in AML-like fish. Red boxes show enlarged details of myeloid and blast cells (×60). (b) Immunofluorescent staining of the myeloid-specific marker Lcp in paraffin-embedded sections confirmed myeloid cells infiltration of the skeletal musculature in c-mybhyper MDS and AML. (c–f) c-mybhyper zebrafish develop ALL in adulthood. (c) May-Grunwald/Giemsa staining of PB cells (upper panels) and KM blood cells (lower panels) obtained from siblings (the left column) and c-mybhyper zebrafish (the right column). Yellow arrows indicate lymphoid blasts in ALL-like fish. Red boxes show enlarged details of lymphoid and blast cells (×60). (d) Immunofluorescent staining of sections confirmed lymphocytes infiltration of the skeletal musculature in c-mybhyper ALL. Expression of T-cell marker tcra (e) or B-cell markers igu and iglc3 (f) in individual siblings and c-mybhyper fish. Numbers in (b) and (d) indicate the number of fish with leukemia infiltration out of total number of fish (Fisher’s exact tests, P<0.05).
As anticipated, the ALL-like c-mybhyper fish showed >80% increase in lymphocytes or >60% increase in precursors with lymphoblasts morphology in the PB, and a >40% increase in lymphocytes in the KM (Figure 4c and Table 2), and likewise, some of them showed pathologic external features, including cachexia, exophthalmos, bleeding and curvature (Supplementary Figures S4G–J). Histological analysis revealed that many non-hematopoietic tissues including the gill, muscle, liver, eye and central nervous system were invaded by lymphocytic cells in the ALL-like c-mybhyper fish (Figure 4d and Supplementary Figure S5B), which are similar to lymphocyte infiltration in ALL human patients. This was further confirmed by using the lymphoid-specific transgenic line rag2-dsRed,26 in which rag2+ cells were found in the head and the anterior half of the body (Supplementary Figure S5C). Examination of T-cell (tcrα, the T-cell receptor) and B-cell (igu and iglc3 for immunoglobins) markers in the PB derived from 10 individual ALL-like c-mybhyper fish revealed that six of the fish expressed a high level of T-cell receptor alpha (tcrα) but not B-cell marker, immunoglobins igu and iglc3 (Figure 4e), whereas the remaining four fish were enriched in the expression of immunoglobins igu and iglc3 but not T-cell marker tcra (Figure 4f). These results indicate that aberrant c-myb hyperactivity can lead to both T-cell and B-cell ALL.
Collectively, these data demonstrate that c-mybhyper fish (10–24 months) can progress to AML and ALL with the incidence of ~ 2.1%, respectively. The occurrence of AML and ALL appears to be age-dependent as the incidence is much lower in 3- to 10-month-old young adult c-mybhyper fish (~0.5%, only 1 out of 203 fish).
c-myb hyperactivity induced zebrafish leukemias are transplantable
To determine the aggressiveness of leukemia induced by c-Myb hyperactivity, whole KM cells from c-mybhyper fish were transplanted into γ-irradiated WT adult hosts and tested whether the MDS, AML and ALL phenotype developed in c-mybhyper fish could be transplanted into WT fish. For MDS, KM cells were harvested from 1-year-old c-mybhyper/lyz-dsRed MDS-like donors or lyz-dsRed controls, in which granulocytes are marked by red fluorescence. Each of the irradiated WT fish received 0.2 million KM cells from 10 independent MDS donors and then raised under normal condition. All survived 21 recipients developed MDS within 2–8 weeks after transplantation as indicated by the infiltration of dsRed+ granulocytes in the periphery (Figure 5a and Supplementary Figure S6A) and the robust expansion of dsRed+ myeloid cells in the KM (with ~ 45% increase of myelomonocytes) (Figures 5b and c and Supplementary Figures S6B and C). In contrast, none of control fish showed any sign of MDS-like phenotype (Figures 5a–c and Supplementary Figures S6B and C). For AML, similar protocol was employed with the exception that the donor KM cells were collected from AML-like c-mybhyper fish (fluorescence labeled c-mybhyper AML fish are unavailable) and WT fish as control. As expected, all three survived recipients received cells from two independent AML donors displayed a significant increase of myeloblasts in the KM within 4–6 weeks after transplantation, whereas controls exhibited normal blood composition (Figures 5d and e). For ALL transplantation, KM cells were collected from 1-year-old c-mybhyper/rag2-dsRed ALL-like donors or rag2-dsRed controls, in which lymphocytes were marked by dsRed. Within 6–9 weeks after transplantation, all six survived recipients received cells from three different ALL donors displayed ALL-like phenotype as indicated by the robust infiltration of rag2+ lymphoblasts, whereas none of controls developed ALL-like phenotype (Figure 5f). These data demonstrate that MDS, AML- and ALL-like leukemic cells in c-mybhyper are transplantable.
Figure 5.
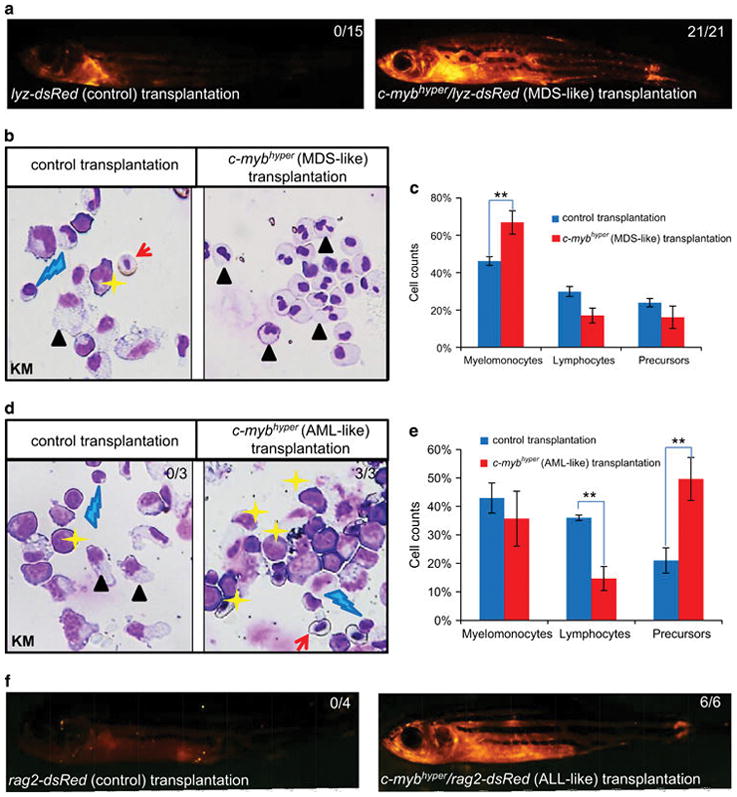
MDS, AML and ALL-like leukemic cells in c-mybhyper zebrafish are transplantable. (a–c) Twenty-one and 15 survived (of 102 and 80) recipients transplanted with MDS-like c-mybhyper/lyz-dsRed and lyz-dsRed control KM cells, respectively. dsRed positive cells repopulated in all survived recipients within 4–8 weeks after transplantation (a). KM cells in fish transplanted with c-mybhyper/lyz-dsRed MDS cells (left panel) and lyz-dsRed KM cells (right panel) were stained by May-Grunwald/Giemsa (b). Blood cell counts of KM were calculated manually based on their morphology (c). (d, e) Three and 3 survived (of 20 and 21) recipients transplanted with ALL-like c-mybhyper and siblings control KM cells, respectively. All survived recipients were stained by May-Grunwald/Giemsa (d). Blood cell counts of KM (e) were calculated manually based on their morphology (KM in fish transplanted with sibling and AML-like c-mybhyper, n =3 and n =3, respectively). (f) Six and 4 survived (of 56 and 25) recipients transplanted with c-mybhyper(ALL-like)/rag2-dsRed and rag2-dsRed control KM cells. dsRed-positive cells repopulation in all survived recipients within 4–8 weeks after transplanted. Numbers in each panel indicate the number of fish with leukemia-like phenotype out of total number of fish (Fisher’s exact tests, P<0.05). The black asterisks indicate statistical differences (t-test; mean ±s.d.; *P<0.05, **P<0.01). Red arrows, yellow asterisks, black arrowheads and blue lightnings indicate erythrocytes, precursors, myelomonocytes and lymphocytes, respectively.
c-mybhyper leukemic model responds to chemotherapeutic drug treatment
Growing evidences have demonstrated that zebrafish is a promising animal model for drug discovery.19 We therefore evaluated the effects of widely used anti-leukemic agent in c-mybhyper fish. For drug treatment, the tolerance of chemotherapeutic was tested and the maximum dose was used (Supplementary Figure S7A). Cytarabine52 is commonly used to treat AML by inhibiting the cell cycle. c-mybhyper embryos and their siblings were incubated with 103 mg/l cytarabine at 1 dpf, and the numbers of SB+ granulocytic cells were calculated in the caudal hematopoietic tissue at 6 dpf (Figures 6a, b and f). Results showed that cytarabine treatment significantly reduced the number of SB+ granulocytes in c-mybhyper embryos (Figures 6b and f), compared with the untreated controls (Figures 6a and f). However, when we performed cytarabine treatment in adult c-mybhyper fish with a 6–20-fold higher than the dose used in mice,31 no curative effect was observed (Supplementary Figure S7B). These results indicate that the cell-cycle-specific antiproliferation drug cytarabine can relieve neoplastic proliferation of granulocytes in c-mybhyper embryos but not in adult fish.
Figure 6.
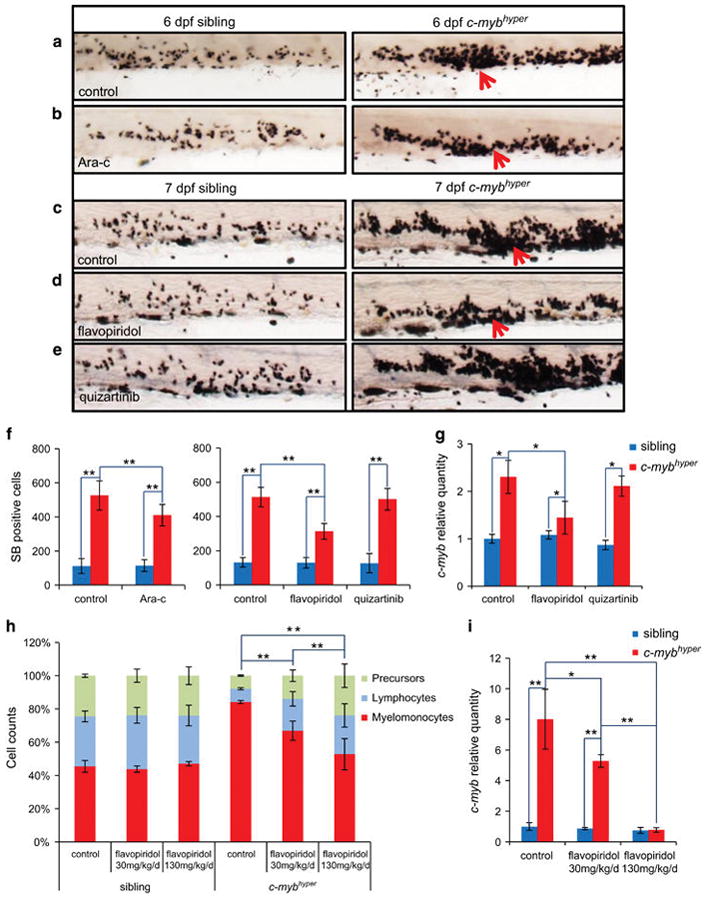
c-mybhyper MDS-like zebrafish respond to chemotherapy. (a–g) Drug treatments in embryos. 1 dpf siblings (left panels) and c-mybhyper (right panels) larvae treated with phosphate-buffered saline control (a) and cytarabine (b) for 5 days and stained with SB at 6 dpf. 1 dpf larvae treated with dimethylsulfoxide (DMSO) control (c), flavopiridol (d) and quizartinib (e) for 6 days and stained with SB at 7 dpf. (f) Average numbers of SB+ cells per larva with drug treatment (t-test, sibling and c-mybhyper, n =14 and n =10, respectively; mean ±s.d.; *P<0.05, **P<0.01). (g) Relative expression of c-myb gene in siblings (blue bar) or c-mybhyper fish (red bar) treated with flavopiridol or quizartinib were examined by qPCR. (t-test, n =30; mean ±s.d.; *P<0.05, **P<0.01). (h) Drug treatments in adult fish. Blood cell counts of 1-year siblings and c-mybhyper KM after intraperitoneal injection with flavopiridol (30 mg/kg/day or 130 mg/kg once daily for 4 days) or phosphate-buffered saline (t-test, n =10; mean ±s.d.; *P<0.05, **P<0.01). (i) Relative expression of c-myb gene in siblings (blue bar) or c-mybhyper adult KM (red bar) treated with flavopiridol was examined by qPCR. (t-test, n =30; mean ±s.d.; *P<0.05, **P<0.01).
To obtain better curative effects, we turned to the c-MYB-targeting drug flavopiridol.53 Maximum tolerance dose (MTD) (0.5 μM) of flavopiridol significantly reduced the numbers of SB+ granulocytes in c-mybhyper embryos compared with the untreated controls (Figures 6c, d and f and Supplementary Figure S7A). As expected, c-myb RNA levels were also significantly reduced in c-mybhyper embryos upon flavopiridol treatment compared with untreated fish (Figure 6g). On the other hand, Flt3-targeting drug quizartinib54 was included as a control and treatment with maximum tolerance dose (0.5 μm) of quizartinib had little effect on the number of SB+ granulocytes and the c-myb RNA levels in c-mybhyper embryos (Figures 6c, e and f and Supplementary Figure S7A). These results indicated that clinically used c-MYB-targeting drug can specifically and effectively reduced the expanded myeloid population in c-mybhyper embryos. To further evaluate the anti-leukemic activity of flavopiridol in adulthood, MDS-like c-mybhyper adult fish were intraperitoneal injected with flavopiridol and phosphate-buffered saline control (once per day) and the number of myeloid cells was quantified 4 days later. Results showed that a low dose (30 mg/kg/day) of flavopiridol was able to significantly reduce the number of myeloid cells in c-mybhyper KM, and a higher dose (130 mg/kg/day) produced a much profound effect (Figure 6h). Likewise, the c-myb level was also significantly reduced in c-mybhyper KM after flavopiridol treatment (Figure 6i). Notably, flavopiridol treatment (both low and high concentrations) had no obvious effect on the number of myeloid cells in control adult fish (Figure 6h). Taken together, the above results demonstrate that the c-MYB-targeting drug flavopiridol can effectively relieve MDS in c-mybhyper embryos and adult fish.
DISCUSSION
For decades, high expression of c-MYB has been found to associate with oncogenic activity and poor prognosis in human AMLs,55 colorectal tumors56 and adenoid cystic carcinomas.57 c-MYB duplication was also identified in 8.4–15% human T-ALL.14,15 The C-terminal truncated c-MYB lacking the negative-regulatory region region was also identified in human angiocentric glioma,58 AML59 and a leukemic cell line established from a chronic myeloid leukemia patient in T-cell blast crisis.16 In this study, we investigated a zebrafish leukemia model with aberrant c-myb hyperactivity. This c-mybhyper model display MDS, AML and ALL phenotypes that resemble the situation of human patients. The neoplastic abnormalities of blood cells in c-mybhyper fish are likely caused by the combined contribution of the duplicated c-Myb-WT and the hyperactive c-Myb-T1, which leads to the upregulation of the same sets of downstream genes including many well-known c-Myb targets involved in cell-cycle progression shown in Table 1, resulting in the development of neoplastic phenotypes. However, we cannot exclude the possibility that the hyperactive c-Myb-T1 may regulate additional sets of the downstream targets distinctive from those of c-Myb-WT and further investigation is required to clarify this issue. Nonetheless, our study demonstrates that c-myb plays a crucial role in leukemogenesis in vivo, and the effective response of this model to chemotherapeutic agents will facilitate future drug evaluation and screening.
MDS is the most common hematologic malignancy affecting the elderly, and patients with MDS often progress to AML or ALL.51 However the mechanisms responsible for the progression from early MDS to ALL or AML remain unclear. The acquisition of additional mutations acts as a factor driving this transition.60 c-mybhyper zebrafish exhibited MDS-like pathological phenotypes from the embryonic stages to adulthood with the subsequent development of AML- or ALL-like symptoms in a small portion of adult fish, with a long latency, similar to the clinical observation of MDS progression to AML or ALL in human patients. MDS phenotypes were found in almost all c-mybhyper fish progeny, and ectopic expression of c-myb-WT and c-myb-T1 contributed to myeloid expansion in WT embryos, suggesting the c-Myb alone is sufficient for the development of MDS; however, the subsequent transformation to an acute leukemia-like phenotype only occurred in some individuals, suggesting c-Myb may be an initiating event for this progression, and clonal evolution or other events were responsible for this progression. The c-myb model thus provides an opportunity to investigate additional risk factors for myeloid and lymphoid leukemias.
Flavopiridol is a MYB-targeting drug that inhibits myb transcriptional elongation.53 Although flavopiridol has been used for the treatment of lymphocytic leukemia in the clinic, its efficacy in relation to myeloid leukemia remains unclear. Some reports have indicated that flavopiridol, combined with other chemotherapeutic drugs, could be used to treat high-risk or drug-resistant patients with myeloid leukemia.61 In our study, overaccumulated granulocytes in the c-mybhyper were largely restored after treatment with flavopiridol, suggesting that it may be effective for myeloid leukemias with aberrant c-myb hyperactivity. These results thus provide new evidence for the clinical application of flavopiridol, as well as confirming the use of the c-mybhyper zebrafish disease model as an in vivo system for investigating new chemotherapeutic drugs.
In summary, this study demonstrated that c-myb hyperactivity in zebrafish leads to MDS, AML and ALL, and that this leukemia animal model responds effectively to clinically used chemotherapeutic drugs. These results suggest that c-Myb may be an attractive therapeutic target in certain types of leukemia, and c-mybhyper zebrafish may provide a valuable in vivo drug-screening model for anti-leukemia drugs.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81470335, 81200340 and 31229003), The Ministry of Science and Technology Project 863 (SS2015AA020309), Team Program of Guangdong Natural Science Foundation (2014A030312002), Talent Recruitment funding and Excellent Young Teacher funding (Yq2013025) of Guangdong Higher Education Institutes and Peal River S&T Nova Program of Guangzhou (2013J2200032).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
WL, MW and ZH designed the research, performed most of the experiments and analyzed the data. JL, JC and TW performed some experiments. AYHL, YL, ZZ, QL, KY, SL and LIZ designed some experiments and revised the manuscript. WL, ZW, YZ and WZ designed the research, analyzed the data and prepared the manuscript.
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
References
- 1.Sheiness D, Gardinier M. Expression of a proto-oncogene (proto-myb) in hemopoietic tissues of mice. Mol Cell Biol. 1984;4:1206–1212. doi: 10.1128/mcb.4.7.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westin EH, Gallo RC, Arya SK, Eva A, Souza LM, Baluda MA, et al. Differential expression of the amv gene in human hematopoietic cells. Proc Natl Acad Sci USA. 1982;79:2194–2198. doi: 10.1073/pnas.79.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lieu YK, Reddy EP. Conditional c-myb knockout in adult hematopoietic stem cells leads to loss of self-renewal due to impaired proliferation and accelerated differentiation. Proc Natl Acad Sci USA. 2009;106:21689–21694. doi: 10.1073/pnas.0907623106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vegiopoulos A, Garcia P, Emambokus N, Frampton J. Coordination of erythropoiesis by the transcription factor c-Myb. Blood. 2006;107:4703–4710. doi: 10.1182/blood-2005-07-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sumner R, Crawford A, Mucenski M, Frampton J. Initiation of adult myelopoiesis can occur in the absence of c-Myb whereas subsequent development is strictly dependent on the transcription factor. Oncogene. 2000;19:3335–3342. doi: 10.1038/sj.onc.1203660. [DOI] [PubMed] [Google Scholar]
- 6.Bender TP, Kremer CS, Kraus M, Buch T, Rajewsky K. Critical functions for c-Myb at three checkpoints during thymocyte development. Nat Immunol. 2004;5:721–729. doi: 10.1038/ni1085. [DOI] [PubMed] [Google Scholar]
- 7.Thomas MD, Kremer CS, Ravichandran KS, Rajewsky K, Bender TP. c-Myb is critical for B cell development and maintenance of follicular B cells. Immunity. 2005;23:275–286. doi: 10.1016/j.immuni.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Lidonnici MR, Corradini F, Waldron T, Bender TP, Calabretta B. Requirement of c-Myb for p210(BCR/ABL)-dependent transformation of hematopoietic progenitors and leukemogenesis. Blood. 2008;111:4771–4779. doi: 10.1182/blood-2007-08-105072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nazarov V, Wolff L. Novel integration sites at the distal 3′ end of the c-myb locus in retrovirus-induced promonocytic leukemias. J Virol. 1995;69:3885–3888. doi: 10.1128/jvi.69.6.3885-3888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grasser FA, Graf T, Lipsick JS. Protein truncation is required for the activation of the c-myb proto-oncogene. Mol Cell Biol. 1991;11:3987–3996. doi: 10.1128/mcb.11.8.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuber J, Rappaport AR, Luo W, Wang E, Chen C, Vaseva AV, et al. An integrated approach to dissecting oncogene addiction implicates a Myb-coordinated self-renewal program as essential for leukemia maintenance. Genes Dev. 2011;25:1628–1640. doi: 10.1101/gad.17269211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegert W, Beutler C, Langmach K, Keitel C, Schmidt CA. Differential expression of the oncoproteins c-myc and c-myb in human lymphoproliferative disorders. Eur J Cancer. 1990;26:733–737. doi: 10.1016/0277-5379(90)90130-l. [DOI] [PubMed] [Google Scholar]
- 13.Machova Polakova K, Lopotova T, Klamova H, Burda P, Trneny M, Stopka T, et al. Expression patterns of microRNAs associated with CML phases and their disease related targets. Mol Cancer. 2011;10:41. doi: 10.1186/1476-4598-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clappier E, Cuccuini W, Kalota A, Crinquette A, Cayuela JM, Dik WA, et al. The C-MYB locus is involved in chromosomal translocation and genomic duplications in human T-cell acute leukemia (T-ALL), the translocation defining a new T-ALL subtype in very young children. Blood. 2007;110:1251–1261. doi: 10.1182/blood-2006-12-064683. [DOI] [PubMed] [Google Scholar]
- 15.Lahortiga I, De Keersmaecker K, Van Vlierberghe P, Graux C, Cauwelier B, Lambert F, et al. Duplication of the MYB oncogene in T cell acute lymphoblastic leukemia. Nat Genet. 2007;39:593–595. doi: 10.1038/ng2025. [DOI] [PubMed] [Google Scholar]
- 16.Tomita A, Watanabe T, Kosugi H, Ohashi H, Uchida T, Kinoshita T, et al. Truncated c-Myb expression in the human leukemia cell line TK-6. Leukemia. 1998;12:1422–1429. doi: 10.1038/sj.leu.2401113. [DOI] [PubMed] [Google Scholar]
- 17.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoletov K, Klemke R. Catch of the day: zebrafish as a human cancer model. Oncogene. 2008;27:4509–4520. doi: 10.1038/onc.2008.95. [DOI] [PubMed] [Google Scholar]
- 19.Ridges S, Heaton WL, Joshi D, Choi H, Eiring A, Batchelor L, et al. Zebrafish screen identifies novel compound with selective toxicity against leukemia. Blood. 2012;119:5621–5631. doi: 10.1182/blood-2011-12-398818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langenau DM, Traver D, Ferrando AA, Kutok JL, Aster JC, Kanki JP, et al. Myc-induced T cell leukemia in transgenic zebrafish. Science. 2003;299:887–890. doi: 10.1126/science.1080280. [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Liu W, Li L, Chen J, Wu M, Zhang Y, et al. Suppression of Pu.1 function results in expanded myelopoiesis in zebrafish. Leukemia. 2013;27:1913–1917. doi: 10.1038/leu.2013.67. [DOI] [PubMed] [Google Scholar]
- 22.North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio) M. Westerfield; Eugene, OR: 1993. [Google Scholar]
- 24.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Jin H, Li L, Qin FX, Wen Z. cMyb regulates hematopoietic stem/progenitor cell mobilization during zebrafish hematopoiesis. Blood. 2011;118:4093–4101. doi: 10.1182/blood-2011-03-342501. [DOI] [PubMed] [Google Scholar]
- 26.Willett CE, Cherry JJ, Steiner LA. Characterization and expression of the recombination activating genes (rag1 and rag2) of zebrafish. Immunogenetics. 1997;45:394–404. doi: 10.1007/s002510050221. [DOI] [PubMed] [Google Scholar]
- 27.Hall C, Flores MV, Storm T, Crosier K, Crosier P. The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev Biol. 2007;7:42. doi: 10.1186/1471-213X-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang K, Huang Z, Zhao L, Liu W, Chen X, Meng P, et al. Large-scale forward genetic screening analysis of development of hematopoiesis in zebrafish. J Genet Genomics = Yi chuan xue bao. 2012;39:473–480. doi: 10.1016/j.jgg.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Jin H, Li L, Xu J, Zhen F, Zhu L, Liu PP, et al. Runx1 regulates embryonic myeloid fate choice in zebrafish through a negative feedback loop inhibiting Pu.1 expression. Blood. 2012;119:5239–5249. doi: 10.1182/blood-2011-12-398362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carradice D, Lieschke GJ. Zebrafish in hematology: sushi or science? Blood. 2008;111:3331–3342. doi: 10.1182/blood-2007-10-052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wunderlich M, Mizukawa B, Chou FS, Sexton C, Shrestha M, Saunthararajah Y, et al. AML cells are differentially sensitive to chemotherapy treatment in a human xenograft model. Blood. 2013;121:e90–e97. doi: 10.1182/blood-2012-10-464677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arguello F, Alexander M, Sterry JA, Tudor G, Smith EM, Kalavar NT, et al. Flavopiridol induces apoptosis of normal lymphoid cells, causes immunosuppression, and has potent antitumor activity In vivo against human leukemia and lymphoma xenografts. Blood. 1998;91:2482–2490. [PubMed] [Google Scholar]
- 33.Sakura H, Kanei-Ishii C, Nagase T, Nakagoshi H, Gonda TJ, Ishii S. Delineation of three functional domains of the transcriptional activator encoded by the c-myb protooncogene. Proc Natl Acad Sci USA. 1989;86:5758–5762. doi: 10.1073/pnas.86.15.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ness SA, Marknell A, Graf T. The v-myb oncogene product binds to and activates the promyelocyte-specific mim-1 gene. Cell. 1989;59:1115–1125. doi: 10.1016/0092-8674(89)90767-8. [DOI] [PubMed] [Google Scholar]
- 35.Sakamoto H, Dai G, Tsujino K, Hashimoto K, Huang X, Fujimoto T, et al. Proper levels of c-Myb are discretely defined at distinct steps of hematopoietic cell development. Blood. 2006;108:896–903. doi: 10.1182/blood-2005-09-3846. [DOI] [PubMed] [Google Scholar]
- 36.Rosson D, Tereba A. Transcription of hematopoietic-associated oncogenes in childhood leukemia. Cancer Res. 1983;43:3912–3918. [PubMed] [Google Scholar]
- 37.Murati A, Gervais C, Carbuccia N, Finetti P, Cervera N, Adelaide J, et al. Genome profiling of acute myelomonocytic leukemia: alteration of the MYB locus in MYST3-linked cases. Leukemia. 2009;23:85–94. doi: 10.1038/leu.2008.257. [DOI] [PubMed] [Google Scholar]
- 38.Lyons SE, Shue BC, Lei L, Oates AC, Zon LI, Liu PP. Molecular cloning, genetic mapping, and expression analysis of four zebrafish c/ebp genes. Gene. 2001;281:43–51. doi: 10.1016/s0378-1119(01)00774-0. [DOI] [PubMed] [Google Scholar]
- 39.Bennett CM, Kanki JP, Rhodes J, Liu TX, Paw BH, Kieran MW, et al. Myelopoiesis in the zebrafish, Danio rerio. Blood. 2001;98:643–651. doi: 10.1182/blood.v98.3.643. [DOI] [PubMed] [Google Scholar]
- 40.Liu F, Wen Z. Cloning and expression pattern of the lysozyme C gene in zebrafish. Mech Dev. 2002;113:69–72. doi: 10.1016/s0925-4773(01)00658-x. [DOI] [PubMed] [Google Scholar]
- 41.Lieschke GJ, Oates AC, Crowhurst MO, Ward AC, Layton JE. Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood. 2001;98:3087–3096. doi: 10.1182/blood.v98.10.3087. [DOI] [PubMed] [Google Scholar]
- 42.Zakrzewska A, Cui C, Stockhammer OW, Benard EL, Spaink HP, Meijer AH. Macrophage-specific gene functions in Spi1-directed innate immunity. Blood. 2010;116:e1–e11. doi: 10.1182/blood-2010-01-262873. [DOI] [PubMed] [Google Scholar]
- 43.Herbomel P, Thisse B, Thisse C. Zebrafish early macrophages colonize cephalic mesenchyme and developing brain, retina, and epidermis through a M-CSF receptor-dependent invasive process. Dev Biol. 2001;238:274–288. doi: 10.1006/dbio.2001.0393. [DOI] [PubMed] [Google Scholar]
- 44.Le Guyader D, Redd MJ, Colucci-Guyon E, Murayama E, Kissa K, Briolat V, et al. Origins and unconventional behavior of neutrophils in developing zebrafish. Blood. 2008;111:132–141. doi: 10.1182/blood-2007-06-095398. [DOI] [PubMed] [Google Scholar]
- 45.Haferlach T, Kern W. Classification and staging of myelodysplastic syndromes. In: Ute H, editor. Hematologic Malignancies: Myelodysplastic Syndromes. Springer; Berlin, Heidelberg: 2006. pp. 39–53. [Google Scholar]
- 46.Muller C, Yang R, Idos G, Tidow N, Diederichs S, Koch OM, et al. c-myb transactivates the human cyclin A1 promoter and induces cyclin A1 gene expression. Blood. 1999;94:4255–4262. [PubMed] [Google Scholar]
- 47.Quintana AM, Liu F, O’Rourke JP, Ness SA. Identification and regulation of c-Myb target genes in MCF-7 cells. BMC cancer. 2011;11:30. doi: 10.1186/1471-2407-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ku DH, Wen SC, Engelhard A, Nicolaides NC, Lipson KE, Marino TA, et al. c-myb transactivates cdc2 expression via Myb binding sites in the 5′-flanking region of the human cdc2 gene. J Biol Chem. 1993;268:2255–2259. [PubMed] [Google Scholar]
- 49.Travali S, Ferber A, Reiss K, Sell C, Koniecki J, Calabretta B, et al. Effect of the myb gene product on expression of the PCNA gene in fibroblasts. Oncogene. 1991;6:887–894. [PubMed] [Google Scholar]
- 50.Garcia P, Clarke M, Vegiopoulos A, Berlanga O, Camelo A, Lorvellec M, et al. Reduced c-Myb activity compromises HSCs and leads to a myeloproliferation with a novel stem cell basis. EMBO J. 2009;28:1492–1504. doi: 10.1038/emboj.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Disperati P, Ichim CV, Tkachuk D, Chun K, Schuh AC, Wells RA. Progression of myelodysplasia to acute lymphoblastic leukaemia: implications for disease biology. Leuk Res. 2006;30:233–239. doi: 10.1016/j.leukres.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 52.Pigneux A, Perreau V, Jourdan E, Vey N, Dastugue N, Huguet F, et al. Adding lomustine to idarubicin and cytarabine for induction chemotherapy in older patients with acute myeloid leukemia: the BGMT 95 trial results. Haematologica. 2007;92:1327–1334. doi: 10.3324/haematol.11068. [DOI] [PubMed] [Google Scholar]
- 53.Mitra P, Pereira LA, Drabsch Y, Ramsay RG, Gonda TJ. Estrogen receptor-alpha recruits P-TEFb to overcome transcriptional pausing in intron 1 of the MYB gene. Nucleic Acids Res. 2012;40:5988–6000. doi: 10.1093/nar/gks286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zarrinkar PP, Gunawardane RN, Cramer MD, Gardner MF, Brigham D, Belli B, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML) Blood. 2009;114:2984–2992. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gopal V, Hulette B, Li YQ, Kuvelkar R, Raza A, Larson R, et al. c-myc and c-myb expression in acute myelogenous leukemia. Leukemia Res. 1992;16:1003–1011. doi: 10.1016/0145-2126(92)90080-q. [DOI] [PubMed] [Google Scholar]
- 56.Biroccio A, Benassi B, D’Agnano I, D’Angelo C, Buglioni S, Mottolese M, et al. c-Myb and Bcl-x overexpression predicts poor prognosis in colorectal cancer: clinical and experimental findings. Am J Pathol. 2001;158:1289–1299. doi: 10.1016/S0002-9440(10)64080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramsay RG, Thompson MA, Hayman JA, Reid G, Gonda TJ, Whitehead RH. Myb expression is higher in malignant human colonic carcinoma and premalignant adenomatous polyps than in normal mucosa. Cell Growth Differ. 1992;3:723–730. [PubMed] [Google Scholar]
- 58.Ramkissoon LA, Horowitz PM, Craig JM, Ramkissoon SH, Rich BE, Schumacher SE, et al. Genomic analysis of diffuse pediatric low-grade gliomas identifies recurrent oncogenic truncating rearrangements in the transcription factor MYBL1. Proc Natl Acad Sci USA. 2013;110:8188–8193. doi: 10.1073/pnas.1300252110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slamon DJ, Boone TC, Murdock DC, Keith DE, Press MF, Larson RA, et al. Studies of the human c-myb gene and its product in human acute leukemias. Science. 1986;233:347–351. doi: 10.1126/science.3014652. [DOI] [PubMed] [Google Scholar]
- 60.Walter MJ, Shen D, Ding L, Shao J, Koboldt DC, Chen K, et al. Clonal architecture of secondary acute myeloid leukemia. N Engl J Med. 2012;366:1090–1098. doi: 10.1056/NEJMoa1106968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karp JE, Blackford A, Smith BD, Alino K, Seung AH, Bolanos-Meade J, et al. Clinical activity of sequential flavopiridol, cytosine arabinoside, and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Leuk Res. 2010;34:877–882. doi: 10.1016/j.leukres.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


