The rhesus macaque model of SIV and Mtb co-infection demonstrated that destruction of lung tissue macrophages promoted the transition from latent to reactivated Mtb. This was reflected by increased monocyte turnover that may be a predictive biomarker of Mtb reactivation.
Keywords: CD4+ T cells, coinfection, HIV
Abstract
Background
Tuberculosis (TB) and human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) profoundly affect the immune system and synergistically accelerate disease progression. It is believed that CD4+ T-cell depletion by HIV is the major cause of immunodeficiency and reactivation of latent TB. Previous studies demonstrated that blood monocyte turnover concurrent with tissue macrophage death from virus infection better predicted AIDS onset than CD4+ T-cell depletion in macaques infected with simian immunodeficiency virus (SIV).
Methods
In this study, we describe the contribution of macrophages to the pathogenesis of Mycobacterium tuberculosis (Mtb)/SIV coinfection in a rhesus macaque model using in vivo BrdU labeling, immunostaining, flow cytometry, and confocal microscopy.
Results
We found that increased monocyte and macrophage turnover and levels of SIV-infected lung macrophages correlated with TB reactivation. All Mtb/SIV-coinfected monkeys exhibited declines in CD4+ T cells regardless of reactivation or latency outcomes, negating lower CD4+ T-cell levels as a primary cause of Mtb reactivation.
Conclusions
Results suggest that SIV-related damage to macrophages contributes to Mtb reactivation during coinfection. This also supports strategies to target lung macrophages for the treatment of TB.
(See the Editorial commentary by Khan and Divangahi, on pages 1675–7.)
Tuberculosis (TB) and human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) continue to be responsible for a significant proportion of deaths caused by infectious disease, especially in developing countries [1, 2]. In 2015, TB was considered to be one of the top 10 causes of death globally. At that time, approximately 10.4 million of the world’s population were diagnosed with new Mycobacterium tuberculosis (Mtb) infections. Of these new Mtb-infected individuals, 11% were also infected with HIV, and 400000 deaths of coinfected persons were reported [3]. Moreover, HIV coinfection is known to be a risk factor for active Mtb infection and also significantly increases the risk for latent TB reactivation [1, 2, 4]. It has been projected that 10% of HIV-infected persons coinfected with latent TB will reactivate each year [5]. Although the mechanisms for TB reactivation are not fully understood, it is widely believed that declining numbers of CD4+ T cells from HIV infection are primarily responsible. However, we previously reported that increasing blood monocyte turnover rates associated with tissue macrophage death better correlated with rapid disease progression to AIDS than did declining levels of CD4+ T cells in rhesus macaques infected with simian immunodeficiency virus (SIV) [6, 7]. Massive SIV infection and apoptosis of the short-lived lung interstitial macrophages (IM) also correlated with AIDS disease progression [8]. Furthermore, the increased monocyte turnover was associated with accumulation of lung IM and pulmonary pathology [9]. Thus, these findings support monocytes and macrophages as major contributors to the pathogenesis of AIDS and pulmonary disease progression in HIV infection. The purpose of this study was to further examine the impact of lung macrophages on the reactivation of TB in the SIV/Mtb coinfection rhesus macaque model.
METHODS
Animals and Inoculations
A total of 36 adult male Indian rhesus macaques (Macaca mulatta) between 4 and 12 years of age were used in the study and housed at the Tulane National Primate Research Center in Covington, Louisiana. Animals were infected with low-dose Mtb via aerosol exposure and subsequently with SIV intravenously as described earlier [10–14]. In brief, concentrated SIVmac239 stock was diluted in Roswell Park Memorial Institute (RPMI) medium, and 300 TCID50 units of virus were injected intravenously. All animal procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals [15] and were approved by the Tulane University Institutional Animal Care and Use Committee.
5-Bromo-2’-deoxyuridine Injection and Sample Collection
The thymidine analog, 5-bromo-2’-deoxyuridine ([BrdU] Sigma-Aldrich, St. Louis, MO), was injected at 60 mg/kg. Bronchoalveolar lavage samples were obtained by rinsing the lung with 2 aliquots of phosphate-buffered saline ([PBS] 20 mL each) under a pediatric fiberoptic bronchoscope. At necropsy, approximately 4 cm3 of lung tissue were obtained and divided for isolation of single-cell suspension, (immuno)histochemistry, and isolation of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA).
Isolation of Macrophages and Lymphocytes From Lung Tissue
Single-cell suspensions from lung tissues (including biopsy and necropsy samples) were prepared using an enzymatic digestion method as previously described [16]. In brief, lung tissues were sliced into 0.5 mm-thick sections after removing bronchi and resuspended in 30 mL RPMI 1640 (Cellgro, Manassas, VA) supplemented with 5% fetal bovine serum ([FBS] catalog no. 26140-079; Gibco, Grand Island, NY), 100 IU/mL penicillin/streptomycin (EMD Millipore, Billerica, MA), 2 mM l-glutamine (Cellgro), 25 mM HEPES (Molecular Biology, Carlsbad, CA), 200 U/mL type IV collagenase (catalog no. 4189; Worthington Biochemical, Lakewood, NJ), and 0.05 mg/mL DNAase I (catalog no. 10104159001; Roche Applied Science, Indianapolis, IN) [17]. The suspensions were then incubated at 37°C for 30 minutes, followed by pipetting, incubation for an additional 10 minutes at 37°C, and enrichment by discontinuous density centrifugation over 24% and 50% Percoll (catalog no. 17-0891-01; GE Healthcare, Boston, MA) at 2000 rpm for 20 minutes (Allegra X-12R; Beckman Coulter, Berea, CA). Cells were recovered from the 24%–50% Percoll interface, washed with 2% PBS-FBS (PBS containing 2% FBS), and stored in liquid nitrogen until further analyses.
Assays
Flow cytometry with a 3-laser FACSAria (Becton Dickinson, San Jose, CA) was applied to analyze the cellular phenotype and incorporation of BrdU. Results were analyzed using FlowJo (version 9.6.2; TreeStar) software. Simian immunodeficiency virus in situ hybridization and anti-Mtb antibody (Cat#ab905, 1:100 dilution) were used to detect virus and Mtb, respectively, in the tissues. Anti-CD163 (1:20; clone 10D6; Novocastra, Buffalo Grove, IL) and anti-BrdU (1:50; clone BU1/75(ICR1); Novus, Littleton, CO) were used to identify macrophages and newly recruited cells.
Confocal microscopy imaging was performed with a Leica TCS SP2 confocal microscope equipped with 3 lasers (Leica Microsystems) at ×400 or ×630 magnification with a resolution of 512 × 512 pixels. Adobe Photoshop software (version 7.0; Adobe Systems) was used to process and assemble the images.
Quantification of SIV RNA in plasma and cell-associated virus DNA in lung tissue was performed using the TaqMan real-time polymerase chain reaction (PCR) method. Cellular DNA extraction from sorted cells or lung tissue was performed using NucleoSpin Tissue (catalog no. 740952; Macherey-Nagel Inc, Bethlehem, PA) following the manufacturer’s instructions. Amplification targeted a 74-base pair fragment in the gag region and was performed in the RT-PCR Unit of the Pathogen Detection and Quantification Core at the Tulane National Primate Research Center. A TaqMan RNase P Control Reagents Kit (catalog no. 4316844; Life Science, Carlsbad, CA) was used to calibrate the cellular input for SIV DNA detection. All real-time PCR assays were carried out using an ABI Prism 7900HT Sequence Detection System (Applied Biosystems). Absolute viral RNA and DNA copy numbers were deduced by comparing the signal strength to corresponding values obtained from six 10-fold dilutions of standardized RNA controls that were reverse transcribed and amplified, or standardized DNA controls, that were amplified in parallel, respectively.
Statistical Analysis
T test or Dunn’s multiple comparisons test following the Kruskal-Wallis test were performed. Spearman’s test was used for correlation analysis. Data were analyzed and graphed using Graphpad Prism 5 software. P < .05 was considered statistically significant.
RESULTS
Increasing Levels of Serum C-Reactive Protein Correlated With the Mycobacterium tuberculosis (Mtb) Burden in Lung Tissues of Macaques at Various Stages of Mtb Infection
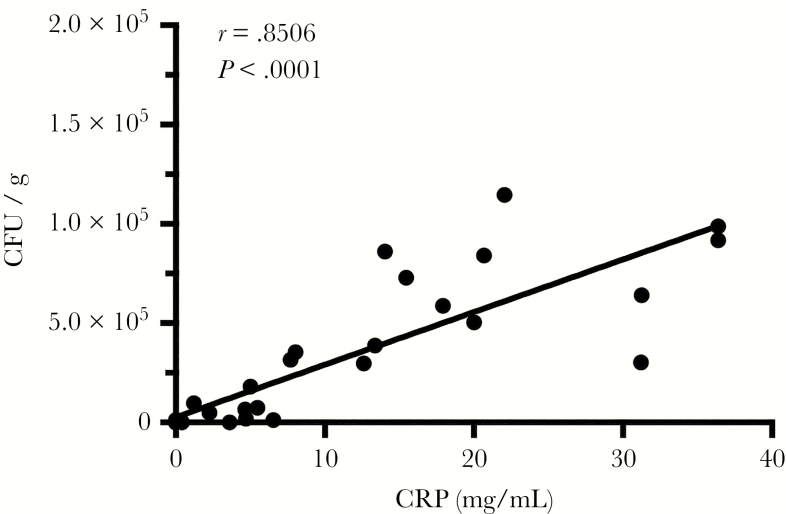
To assess whether pulmonary Mtb-infection was associated with a systemic inflammatory response in the rhesus macaques, we correlated Mtb levels in the lung tissue with serum C-reactive protein (CRP) levels. In our model, low-dose aerosol transmission produced asymptomatic latent Mtb infection (LTBI) in rhesus macaques. Subsequently, intravenous (i.v.) inoculation with pathogenic SIVmac239 was performed to study reactivation of clinically overt TB. Results from a tuberculin skin test (TST) and PRIMAGAM (data not shown) confirmed that all animals became TB infected after aerosol exposure to Mtb. Delineation between active TB (ATB) and LTBI in humans relies on radiology (ie, chest x-ray [CXR]), as well as microbiological culture of body fluids from the TST-positive patients, but interpretation can be ambiguous due to inaccuracy in measuring the magnitude of Mtb replication, especially in the lungs of the infected individuals. Increasing body temperature, declining body weight, and increasing levels of serum CRP are frequently observed during clinically overt TB in humans [18]. In our experimental aerosol-infected macaques, the concentrations of serum CRP statistically significantly correlated with the relative bacterial burdens (r = 0.85006, P < .0001; Figure 1) in lung tissues at various stages of Mtb infection ranging from latent to ATB. Therefore, serum CRP concentrations were used to categorize the TB status of Mtb-infected macaques, with or without SIV coinfection, in these studies. Animals that exhibited TB reactivation due to SIV coinfection had Mtb burdens ranging from 103 to 104 colony formation units (CFU) per gram of lung tissue, whereas coinfected animals that remained latent harbored significantly lower Mtb burdens (100–102 CFU/gram) similar to levels reported in animals with LTBI [10]. Increasing body temperature, declining body weight, and increasing levels of serum CRP were frequently observed during clinically overt TB in our SIV/TB macaque model [19].
Figure 1.
Increasing levels of serum C-reactive protein (CRP) correlated with the Mycobacterium tuberculosis (Mtb) burden in lung tissues of infected macaques. Spearman’s correlation analysis was performed to relate the Mtb bacterial load of the lung (CFU/gram) and the level of serum CRP (mg/dL) of the Mtb singly-infected macaques with different clinical manifestations (n = 23).
Simian Immunodeficiency Virus Infection Reactivates Latent Tuberculosis in Rhesus Macaques
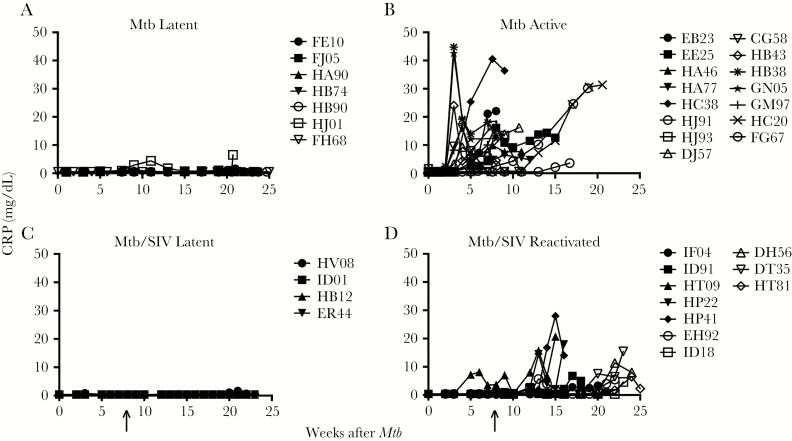
We organized the 22 Mtb-infected animals (Figure 2A and B) and 14 Mtb/SIV-coinfected animals (Figure 2C and D) according to their longitudinal serum CRP levels as latent without or with SIV (Figure 2A and C) and with ATB or reactivated TB after SIV infection (Figure 2B and D). All 36 Mtb-infected rhesus macaques received either a low dose of Mtb (200 CFU) or a high dose of Mtb (>200 but <5000 CFU). The Mtb infection was confirmed in all exposed macaques using TST and PRIMAGAM tests as previously described [10]. Similar to results from our previous study [20], the majority of macaques (~78%) exposed with low-dose Mtb (200 CFU) remained latent throughout our study without any sign of ATB and with low or undetectable plasma CRP levels throughout this time course (Figure 2A). In contrast, 100% of the high-dose Mtb-exposed macaques exhibted clinical signs indicative of ATB and expressed increased levels of plasma CRP as early as 2 weeks postinfection, supporting the use of CRP levels to differentiate between active versus latent TB (Figure 2B). For the next study, we thus exposed an additional 14 adult macaques to a low-dose aerosol Mtb inoculum (Figure 2C and D), and these animals maintained low levels of serum CRP for at least 8 weeks, consistent for latent TB. We then also infected these 14 macaques with i.v. inoculation of pathogenic SIVmac239. Of these Mtb/SIV-coinfected macaques, 4 (~29%) remained latent. The other 10 animals (~71%) exhibited increased serum CRP concentrations diagnostic of ATB (Figure 2D), developed fever and weight loss, and required euthanasia at time points as indicated (Figure 2B). Furthermore, these clinical parameters correlated with total lung bacterial burden at euthanasia (data not shown). This experimental design established an Mtb/SIV coinfection nonhuman primate (NHP) model to study mechanisms of TB reactivation after SIV infection (Supplementary Figure 1). Furthermore, this demonstrated that SIV infection helped promote TB reactivation after low-dose Mtb infection (ie, from ~22% with low-dose Mtb vs ~71% with low-dose Mtb/SIV coinfection).
Figure 2.
Latent Mycobacterium tuberculosis (Mtb) infection is reactivated by coinfection with simian immunodeficiency virus (SIV). (A) The majority of macaques (7 of 9) administered low-dose aerosol Mtb remained latent and exhibited extremely low levels of serum C-reactive protein (CRP) (n = 7). (B) Macaques with signs of active tuberculosis (TB) had high levels of serum CRP (n = 15). (C and D) Nonhuman primate (NHPs) latently infected with Mtb as in group A with no clinical signs of TB were infected with SIV (arrow). Despite SIV infection, 4 Mtb/SIV-coinfected NHPs in group C did not show signs of TB reactivation in contrast to animals in group D (n = 10) that required euthanasia after presenting with active TB.
Increasing Blood Monocyte Turnover Rate in Mycobacterium tuberculosis/Simian Immunodeficiency Virus-Coinfected Rhesus Macaques Correlates With Reactivation of Latent Tuberculosis
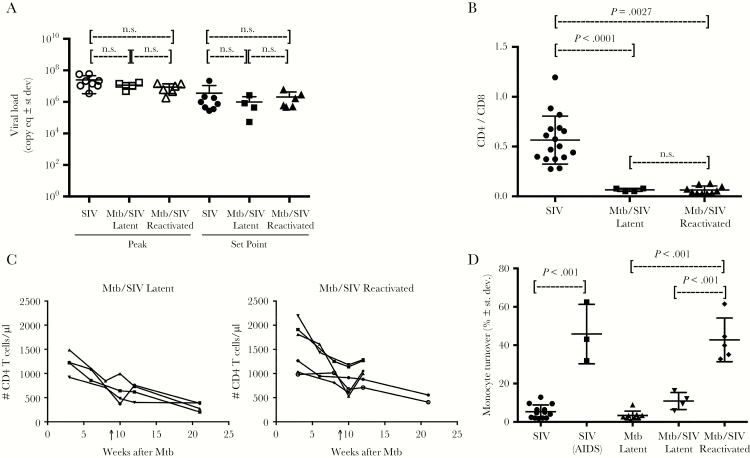
To determine whether plasma viral load is associated with TB activation, we compared the level of acute phase peak viral load (PVL) and subsequent set point viral load (SVL) between SIV-infected monkeys with LTBI (LTBI/SIV) and SIV-infected monkeys with reactivated Mtb (ATB/SIV), as well as in monkeys only infected with SIV as reference controls. The results demonstrated that there were no significant differences in PVL and SVL among the 3 groups of animals (Figure 3A). This suggested that Mtb infection did not affect SIV replication capacity directly, regardless of latent or reactivated Mtb infection status, corroborating results from our previous studies [10]. Depletion of CD4+ T cells is generally considered a main cause of immunodeficiency that contributes to TB reactivation in Mtb/HIV-coinfected humans. To address this, we first evaluated CD4+ T-cell levels in the lung of the Mtb-infected macaques, with and without SIV coinfection. We observed significantly lower CD4+ T-cell levels in the lungs of Mtb/SIV-coinfected animals compared with those infected with Mtb alone (Figure 3B). However, the CD4+ T levels were similar between the SIV-infected animals with ATB and LTBI (Figure 3B). In fact, the levels of CD4+ T cells in the lungs of both LTBI/SIV and ATB/SIV groups decreased at similar rates in lung (Figure 3B) as well as in the blood (Figure 3C), thus repudiating reduction of CD4+ T cells as the primary inducer of Mtb reactivation. Measures of CD4 T-cell depletion also were similar between tissue samples obtained from both granuloma and nongranuloma areas of the lung (Supplementary Figure 2).
Figure 3.
Increased turnover levels of blood monocytes in the Mycobacterium tuberculosis (Mtb)/simian immunodeficiency virus (SIV)-coinfected rhesus macaques correlate with reactivation of latent tuberculosis (TB). (A) Analyses were performed to compare peak plasma viral loads and set point viral loads among 3 groups of SIV-infected rhesus macaques (SIV infection alone, n = 8; latent Mtb/SIV infection, n = 4; and reactivated Mtb/SIV coinfection, n = 6) during the acute stage viral peak (left) and after viral set point (right). (B) Depletion of CD4+ T cells in the lung was evaluated using CD4/CD8 ratios (Mtb infection alone, n = 17; latent Mtb/SIV coinfection, n = 4; and reactivated Mtb/SIV coinfection, n = 6). (C) The absolute number of CD4+ T cells in blood was calculated based on complete blood count and flow cytometry data (latent Mtb/SIV coinfection, n = 4; and reactivated Mtb/SIV coinfection, n = 6). Arrows depict time of SIV infection. (D) Monocyte turnover was measured by detecting 5-bromo-2’-deoxyuridine (BrdU)-labeled monocytes (blood) 24 hours after in vivo BrdU injection. Abbreviations: AIDS, acquired immune deficiency syndrome; n.s., not significant; st dev, standard deviation.
Next, we evaluated monocyte kinetics after in vivo BrdU incorporation, and we found that monocyte turnover rates from the blood were significantly higher in the SIV-infected macaques with reactivated TB than in the SIV-infected macaques that maintained LTBI (P < .001; Figure 3D) despite the similarly high SIV viral loads (Figure 3A). In addition, this higher level of monocyte turnover observed in the ATB/SIV animals was similar to the rate observed in SIV-infected monkeys progressing to AIDS (Figure 3D). It is interesting to note that the LTBI/SIV monkeys exhibited significantly lower monocyte turnover rates compared with ATB/SIV, which were instead similar to those observed in asymptomatic cases of SIV as well as LTBI (Figure 3D). Thus, the increased monocyte turnover appeared to be associated with TB reactivation but not SIV viral load or CD4+ T-cell levels.
Increased Turnover Rate of Lung Macrophages in Mycobacterium tuberculosis/Simian Immunodeficiency Virus-Coinfected Rhesus Macaques Correlates With Reactivation of Latent Tuberculosis
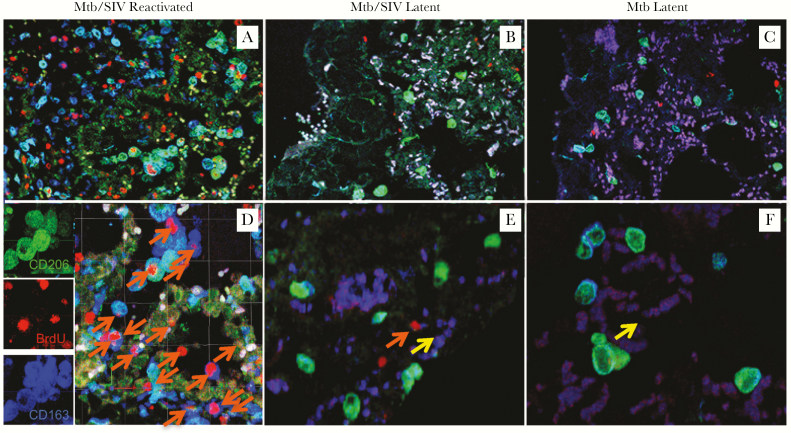
Increased monocyte turnover was associated with severity of lung tissue damage and disease progression in SIV-infected macaques [9]. To evaluate dynamics of tissue macrophages during Mtb reactivation in the SIV-coinfected monkeys, we used in vivo BrdU labeling to monitor cell division and turnover just before necropsy, as previously described in the lung of SIV-infected macaques [8]. In that study, we demonstrated that the level of BrdU+ tissue macrophages correlated directly with an increase in recently divided monocytes into the lung that differentiated into IM, thus illustrating BrdU positivity in lung macrophages as an indicator for tissue macrophage turnover. Moreover, this increase of tissue macrophage turnover also correlated with increased apoptosis measured with TUNEL [8, 20]. In this study, we show that more CD163+CD206− IM stained for BrdU in lung of the ATB/SIV macaques (Figure 4A and D) compared with that of the lungs from the LTBI/SIV-coinfected macaques (Figure 4B and E) or LTBI only animals (Figure 4C and F). We confirmed these observations with flow cytometry analysis of single cells obtained from the uninfected and infected macaques with varying degrees of monocyte turnover (Supplementary Figure 3).
Figure 4.
Increased turnover of lung macrophages in the Mycobacterium tuberculosis (Mtb)/simian immunodeficiency virus (SIV)-coinfected rhesus macaques correlates with reactivation of latent tuberculosis (TB). (A–F) Confocal microscopy was used to analyze lung tissue sections stained with anti-CD206 (mature macrophages; green), anti-5-bromo-2’-deoxyuridine (BrdU) (turnover; red), and anti-CD163 (macrophages; blue), in Mtb/SIV-coinfected macaques with signs of TB reactivation (A and D, n = 2), Mtb/SIV-coinfected animals that remained latent (B and E, n = 2), as well as from the lungs of macaques infected only with Mtb that remained latent (C and F, n = 2). Images were captured from the capsid region of granuloma with a Leica TCS SP2 confocal microscope equipped with 3 lasers (Leica Microsystems) at ×200 (A, B, C) or ×630 (D, E, F) magnification. Orange arrows indicate recently immigrated macrophages ([24]), and yellow arrows indicate macrophages without BrdU label.
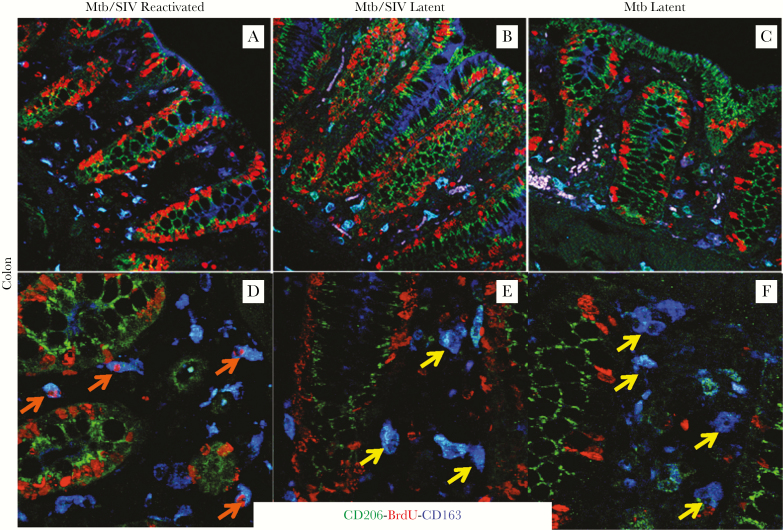
Increased Turnover Rate of Intestinal Macrophages in the Mycobacterium tuberculosis/Simian Immunodeficiency Virus-Coinfected Rhesus Macaques Also Correlates With Reactivation of Latent Tuberculosis
To further test the hypothesis that SIV infection affects the turnover of macrophages that then contributes to Mtb reactivation systemically, macrophage turnover in the colon was evaluated by triple immunofluorescence staining (BrdU, CD163, and CD206) and confocal imaging. As shown in Figure 5B and E, colon tissue from the SIV-infected macaques with LTBI demonstrated relatively intact tissue structure and low macrophage turnover based on a lack of BrdU+CD163+ macrophages comparable to that observed in Mtb-infected macaques without concurrent SIV infection (Figure 5E and F, yellow arrows). In contrast, colon tissue from the ATB/SIV macaques (Figure 5A and D, orange arrows) exhibited more severe tissue damage and higher numbers of BrdU+CD163+ macrophages comparable to what is observed during advanced stages of SIV infection and AIDS (data not shown).
Figure 5.
Increased turnover of intestinal macrophages in Mycobacterium tuberculosis (Mtb)/simian immunodeficiency virus (SIV)-coinfected rhesus macaques also correlates with reactivation of latent tuberculosis (TB). Paraffin-embedded colon tissues collected during necropsy were stained as described in Figure 4 with anti-CD206 (mature macrophages; green), anti-5-bromo-2’-deoxyuridine (BrdU) (turnover; red), and anti-CD163 (macrophages; blue) antibodies and examined by confocal imaging. Images of intestinal tissues are shown from Mtb/SIV-coinfected macaques with signs of TB reactivation (A and D, n = 2), Mtb/SIV-coinfected animals that remained latent (B and E, n = 2), and from macaques infected only with Mtb that remained latent (C and F, n = 2). Images were captured at ×200 (A, B, C) or ×630 (D, E, F) magnification. Orange arrows indicate recently immigrated macrophages, and yellow arrows indicate macrophages without BrdU label.
Increased Simian Immunodeficiency Virus (SIV) Levels Are Observed in Lung Tissue of Rhesus Macaques With Reactivated Mycobacterium tuberculosis/SIV
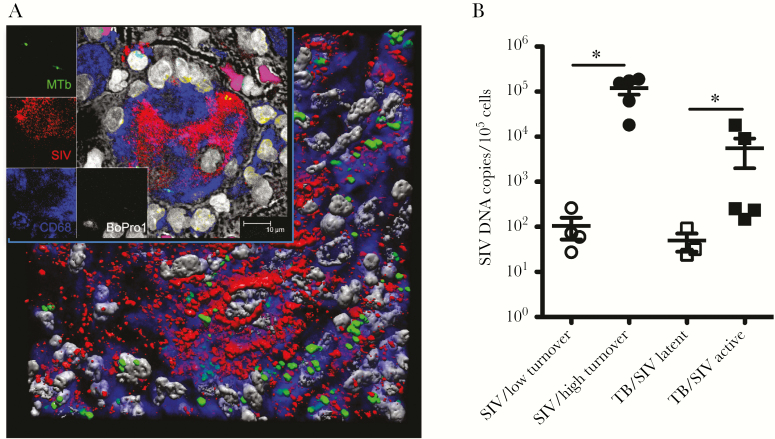
We also reported previously that massive SIV infection and increased death rate of lung IM positively correlated with elevated monocyte turnover and progression to AIDS [8]. Moreover, SIV RNA was visualized by confocal microscopy in lung macrophages of the SIV-infected animals exhibiting high monocyte turnover. In these preliminary results, SIV was also detected in lung tissues of ATB/SIV macaques, whereas lower or undetectable levels of SIV RNA were detected in the lung tissues of LTBI/SIV and LTBI monkeys, respectively (data not shown). We previously showed that Mtb and SIV colocalize in cells in the lung [10], and here we further demonstrate that the cells Mtb and SIV coinfected are macrophages (Figure 6A). To further confirm that the level of SIV infection promotes the increased macrophage turnover in ATB/SIV monkeys, we quantified SIV DNA levels from whole lung tissues. As shown in Figure 6B, the levels of SIV DNA in the lung were low in SIV-infected NHPs that presented with lower monocyte turnover as well as in Mtb/SIV-coinfected NHPs that remained latent. In contrast, significantly higher levels of SIV DNA were detected in the lung of ATB/SIV macaques that exhibited higher monocyte turnover.
Figure 6.
Increased simian immunodeficiency virus (SIV) levels in lung tissue of rhesus macaques with Mycobacterium tuberculosis (Mtb) (reactivated)/SIV. (A) Confocal microscopy was used to analyze stained lung tissue sections from Mtb/SIV-coinfected macaques with antibodies specific to Mtb (green), SIV RNA (red), macrophage marker CD68 (blue), and BoPro1 for cellular nuclei (gray) to identify Mtb/SIV-coinfected lung macrophages (n = 3). (B) The number of SIV deoxyribonucleic acid (DNA) copy equivalents in lung tissue was quantified with TaqMan real-time polymerase chain reaction. Student’s t test was applied for comparing the SIV DNA copy number in the lung tissue from macaques with active TB/SIV (n = 5), from macaques with latent TB/SIV infection (n = 3), and from SIV-infected macaques exhibiting low monocyte turnover rate (≤30%, n = 4) and high monocyte turnover rate (>30%, n = 5). P < .05 was considered significant (*).
DISCUSSION
Lung diseases including chronic obstructive pulmonary disease, interstitial lung disease, pulmonary arterial hypertension [21], cavitary lung disease [22], fibrosis, and neoplasms [21] are also associated with late-stage HIV infection. In previous studies, we demonstrated that increasing blood monocyte turnover rates resulting from death of lymph node tissue macrophages better predicted onset of disease progression to AIDS in SIV-infected macaques than did declining blood CD4+ T-cell levels [6]. Furthermore, increased monocyte turnover was associated with severity of lung tissue damage in SIV-infected macaques [9]. In a subsequent study, we also demonstrated that macrophages of the lung were similarly affected by SIV during AIDS progression [8]. Together, these data suggested that macrophages play a crucial role in the pathogenesis of AIDS in SIV-infected rhesus macaques, particularly in the case of lung tissue damage observed during AIDS progression.
In the present study, we sought to determine the role of lung macrophages in the reactivation of latent TB after SIV infection. Accurate measurement of Mtb replication in the lung during TB disease is difficult. Chest x-ray or sputum culture could be used to diagnose ATB; however, distinguishing different degrees of Mtb replication during transitions between LTBI and ATB is difficult. Therefore, we examined possible markers that could well reflect the degree of Mtb replication in the lung of Mtb-infected macaques. As shown in Figure 1, the level of CRP in plasma correlated very well with Mtb levels calculated as CFU/gram of lung at different stages of TB disease. C-reactive protein may not be a reliable marker to fully monitor Mtb infection and disease status in humans because it is considered as a nonspecific indicator of acute or chronic inflammation. In this rhesus macaque model of Mtb/SIV coinfection, however, we identified monocyte turnover rate as a reliable marker of TB disease progression with corresponding increased CRP levels during reactivation and rarely detected elevated CRP levels in control uninfected macaques (data not shown).
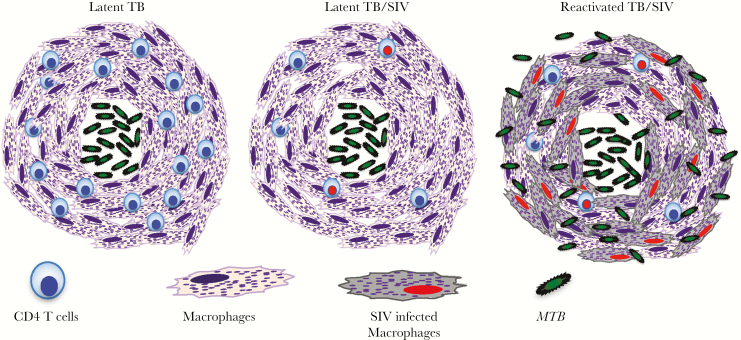
As previously shown [10], the majority of macaques exposed to aerosol low-dose (~200 CFU) and high-dose (~5000 CFU) Mtb developed LTBI and ATB, respectively, and disease presentation correlated with the CXR scores and weight loss (Figure 2A and B). Therefore, we used animals administered an aerosol low dose of Mtb producing positive TST and PRIMAGAM results and low CRP levels (LTBI) for infection with SIV to evaluate the development of ATB, as described previously [10]. We were surprised to find that not all of the SIV-infected macaques (10 of 14) developed ATB at the end of the study. Thus, we took advantage of this unique group of monkeys with LTBI despite SIV infection to decipher the mechanism of TB activation. We observed high levels of SIV DNA in the lung macrophages of Mtb (reactivated)/SIV-coinfected macaques but not in the Mtb (latent)/SIV-coinfected macaques. CD4+ T-cell loss was similar in both groups with latent and ATB, but there were striking differences in monocyte and macrophage turnover rates that were higher in the animals with reactivation compared with those that remained asymptomatic. These findings suggested that SIV infection of tissue macrophages could directly promote macrophage cell death via apoptosis, as we previously reported in macrophages of SIV-infected macaques using TUNEL [8, 20]. We propose that the monocyte turnover rate is an indicator of tissue macrophage (short-lived) turnover. Our model for data in the present study (Figure 7) suggests that the macrophages comprising an important part of the granulomas during TB infection are continuously fed by new macrophages derived from circulating monocytes to maintain the integrity of the granuloma structure. Therefore, as SIV starts infecting macrophages, including those of the granulomas, the integrity of the granuloma structure may be altered and that this causes Mtb to leak and spread from the granulomas. Thus, we consider that monocyte turnover reflects tissue macrophage destruction (including the macrophages of the granulomas) resulting from SIV infection. Consequently, breakdown of the granuloma structure containing Mtb leads to the reactivation of TB after SIV infection. In fact, TB reactivation after SIV infection could also be considered as an indicator of SIV/HIV disease progression. Measured levels of monocyte and macrophage turnover rates in blood, lung, and gut tissues reflected systemic and local tissue differences in monocyte and macrophage populations, between SIV-infected groups with latent or reactivated TB. Taken together, results from the previous and present studies suggest that increased SIV virus infection kills tissue macrophages, which in turn promotes increased blood monocyte turnover to generate cells for replacement of the destroyed tissue macrophages, and that this damage to pulmonary macrophages likely contributes to reactivation of latent Mtb.
Figure 7.
Proposed mechanism of tuberculosis (TB) reactivation of SIV-infected macaques. Mycobacterium tuberculosis (Mtb) organisms are known to be contained by the formation of granulomas that mainly comprise macrophages and T cells including CD4+ T cells (latent TB). Soon after simian immunodeficiency virus (SIV) infection, CD4+ T cells declined regardless of the TB latency or reactivation status. In this model, massive infection of macrophages by SIV and a high rate of cell death correlating with increased blood monocyte turnover were associated with TB reactivation in the Mtb/SIV-coinfected macaques. Macrophage destruction from SIV infection thus appears to be critical for TB reactivation more than CD4+ T-cell decline in human immunodeficiency virus-infected individuals with latent TB.
CONCLUSIONS
Our results also suggest that mechanisms associated with increased monocyte turnover during TB/AIDS reactivation in the NHP model seem to be different from immune activation that was described for lymphocytes [23–26]. Our data suggest that the progressive damage to monocytes/macrophages during SIV infection independently or during coinfection with Mtb in NHPs (and presumably Mtb/HIV coinfection in humans) leads to attempts by the immune system to re-establish homeostasis and replace damaged tissue macrophages. As a result, the increase in monocyte/macrophage turnover rates appear to dictate the tempo of disease progression with reactivation of LTBI more consistently than does only the loss of CD4+ T cells. In other words, investigations about the Mtb/SIV coinfection model in rhesus macaques indicated that the depletion of activated CD4+ T cells was insufficient to explain the reactivation of LTBI, which, instead, correlated better with infection and turnover of IM in the lung. It is still possible that the decline in CD4+ T cells (1) gradually affects homeostasis and the functional properties of myeloid cells including macrophages and (2) contributes to the reactivation of latent TB after HIV/SIV infection. Subsequent studies are still necessary to delineate the molecular and the cellular mechanisms of increased macrophage turnover and LTBI reactivation, but the present results suggest that macrophages should be considered as targets for treatment strategies.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Toni P. Penny, Desiree K. Waguespack, Ashley N. Leach, Erin M. Haupt, Julie Bruhn, and Calvin Lanclos in the Division of Immunology and Dr. Jason P. Dufour in the Division of Veterinary Medicine at Tulane National Primate Research Center for technical and clinical assistance.
Author contributions. M. J. K. and D. K. participated in the study design, experimental research, data analysis, data interpretation, and manuscript preparation; C. S., Y. C., and S. M. contributed equally in the study design, experimental research, and data analysis of the manuscript; M. A. participated in studies of simian immunodeficiency virus (SIV) deoxyribonucleic acid and ribonucleic acid quantification study; C. C. M. participated in SIV immunofluorescent staining study; X. A. participated in the confocal imaging and interpretation of results; C.J.R. participated in performing the aerosol infection. K. M. M. and E. S. D. participated in data interpretation and preparation of the manuscript.
Financial support. This work was funded by grants from the National Institutes of Health AI097059, AI110163, and HL125054 to M. J. K., AI089323 and HL106790 to D. K., and OD011104 to the Tulane National Primate Research Center
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis 2010; 50(Suppl 3):S201–7. [DOI] [PubMed] [Google Scholar]
- 2. Pawlowski A, Jansson M, Sköld M, Rottenberg ME, Källenius G. Tuberculosis and HIV co-infection. PLoS Pathog 2012; 8:e1002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Global Tuberculosis Report 2016. Geneva, Switzerland, 2016. [Google Scholar]
- 4. Corbett EL, Watt CJ, Walker N, et al. . The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med 2003; 163:1009–21. [DOI] [PubMed] [Google Scholar]
- 5. Montales MT, Chaudhury A, Beebe A, Patil S, Patil N. HIV-associated TB syndemic: a growing clinical challenge worldwide. Front Public Health 2015; 3:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hasegawa A, Liu H, Ling B, et al. . The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood 2009; 114:2917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burdo TH, Soulas C, Orzechowski K, et al. . Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog 2010; 6:e1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cai Y, Sugimoto C, Arainga M, et al. . Preferential destruction of interstitial macrophages over alveolar macrophages as a cause of pulmonary disease in simian immunodeficiency virus-infected rhesus macaques. J Immunol 2015; 195:4884–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cai Y, Sugimoto C, Liu DX, et al. . Increased monocyte turnover is associated with interstitial macrophage accumulation and pulmonary tissue damage in SIV-infected rhesus macaques. J Leukoc Biol 2015; 97:1147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mehra S, Golden NA, Dutta NK, et al. . Reactivation of latent tuberculosis in rhesus macaques by coinfection with simian immunodeficiency virus. J Med Primatol 2011; 40:233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dutta NK, Mehra S, Didier PJ, et al. . Genetic requirements for the survival of Tubercle bacilli in primates. J Infect Dis 2010; 201:1743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mehra S, Pahar B, Dutta NK, et al. . Transcriptional reprogramming in nonhuman primate (rhesus macaque) tuberculosis granulomas. PLoS One 2010; 5:e12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dutta NK, Mehra S, Martinez AN, et al. . The stress-response factor SigH modulates the interaction between Mycobacterium tuberculosis and host phagocytes. PLoS One 2012; 7:e28958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luo Q, Mehra S, Golden NA, Kaushal D, Lacey MR. Identification of biomarkers for tuberculosis susceptibility via integrated analysis of gene expression and longitudinal clinical data. Front Genet 2014; 5:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Council NR. Guide for the Care and Use of Laboratory Animals: Eighth Edition.Washington, DC: The National Academies Press, 2011. [Google Scholar]
- 16. Cai Y, Sugimoto C, Arainga M, Alvarez X, Didier ES, Kuroda MJ. In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: implications for understanding lung disease in humans. J Immunol 2014; 192:2821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gorden KK, Qiu XX, Binsfeld CC, Vasilakos JP, Alkan SS. Cutting edge: activation of murine TLR8 by a combination of imidazoquinoline immune response modifiers and polyT oligodeoxynucleotides. J Immunol 2006; 177:6584–7. [DOI] [PubMed] [Google Scholar]
- 18. Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic and prognostic value of serum C-reactive protein for screening for HIV-associated tuberculosis. Int J Tuberc Lung Dis 2013; 17:636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Foreman TW, Mehra S, LoBato DN, et al. . CD4+ T-cell-independent mechanisms suppress reactivation of latent tuberculosis in a macaque model of HIV coinfection. Proc Natl Acad Sci U S A 2016; 113:E5636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sugimoto C, Merino KM, Hasegawa A, et al. . Critical role for monocytes/macrophages in rapid progression to AIDS in pediatric simian immunodeficiency virus-infected rhesus macaques. J Virol 2017; 91; pii: e00379-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Almodovar S, Hsue PY, Morelli J, Huang L, Flores SC. Pathogenesis of HIV-associated pulmonary hypertension: potential role of HIV-1 Nef. Proc Am Thorac Soc 2011; 8:308–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aviram G, Fishman JE, Sagar M. Cavitary lung disease in AIDS: etiologies and correlation with immune status. AIDS Patient Care STDS 2001; 15:353–61. [DOI] [PubMed] [Google Scholar]
- 23. Diedrich CR, Flynn JL. HIV-1/mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis?Infect Immun 2011; 79:1407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Russell DG, Barry CE 3rd, Flynn JL. Tuberculosis: what we don’t know can, and does, hurt us. Science 2010; 328:852–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin PL, Rutledge T, Green AM, et al. . CD4 T cell depletion exacerbates acute Mycobacterium tuberculosis while reactivation of latent infection is dependent on severity of tissue depletion in cynomolgus macaques. AIDS Res Hum Retroviruses 2012; 28:1693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Diedrich CR, Mattila JT, Klein E, et al. . Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PLoS One 2010; 5:e9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.