Abstract
The environmental estrogen, zearalenone (ZEA), is found in the food supply from Fusarium fungal contamination in grains and sometimes used as a growth promoter for beef cattle. Long-term exposure to ZEA and its metabolites may present health risk due to higher estrogenic activity. Serum ZEA metabolites were measured to determine the exposure and the association with food intake in 48 overweight/obese women (52 ± 9 years). The free and conjugated ZEA indicated the highest detection rate of all the metabolites. Conjugated ZEA and total ZEA metabolites were lower (p=0.02) in overweight/obese than normal weight women, and free metabolites were either the same or showed a trend to be higher. In addition, those with highest (280–480 g/d) compared those with lowest (<115 g/d) meat consumption had higher conjugated serum ZEA metabolite concentrations (p<0.05). Intakes of other food groups (i.e., dairy, cereal, etc.) were not associated with ZEA metabolites. These findings indicate that ZEA and its metabolites are detectable in nearly all women and concentrations are associated with greater meat intake, and influenced by body mass index. Determining how the food supply influences human concentrations of ZEA metabolites is warranted, as well as determining vulnerable populations.
Keywords: Body mass index, diet, estrogen disruptor, mycoestrogen, zearalenone
1. Introduction
Zearalenone (ZEA, also known as ZEN) is a nonsteroidal mycotoxin produced as a secondary metabolite by numerous species of Fusarium fungi found across continents, contaminating human foods and grains fed to livestock [1, 2]. Zeranol (α-ZAL), a synthetic derivative of ZEA, is a Food and Drug Administration approved growth promoter for use in beef cattle that is banned in the European Union [3]. ZEA and its metabolites (ZEA metabolites) have been classified as mycoestrogens, phytoestrogens, and/or growth promoters and are chemically similar to the catechols of endogenous 17-β estradiol and estrone [3, 4] (See Supplemental Figure 1). After being ingested with food, ZEA is rapidly absorbed and initially metabolized by the intestine and liver into its major biologically active metabolites, α- and β-zearalenol. In animal models, ZEA exposure has been associated with reproductive dysfunction, cancer and altered immune function, possibly due to its estrogenic activity and the binding affinity of ZEA (and its metabolites) to estrogen receptors [1, 5, 6].
However, very few in vivo studies specifically investigating Fusarium mycotoxins, including ZEA, have been performed in humans [7]. A study examining the relationship between urinary mycoestrogens, breast development, and menarche found measurable levels of ZEA and its associated metabolites in girls, ages 9–10 years [8]. Girls having detectable ZEA levels in their urine were found to be shorter and less likely to have reached the onset of breast development [8]. Dietary beef and popcorn intake were found to be associated with higher urinary ZEA in the girls. Additionally, a case study of a healthy male, showed that a high compared to no cereal diet, increased urinary ZEA excretion [9]. Both urinary and serum ZEA have also been reported in pregnant women [10]. Other mycotoxin exposure studies in different countries also reported concurrent exposure of multiple mycotoxins in their study populations [11–14]. Higher urinary excretion rate of ZEA suggests exposure in persons is attributed to contaminated maize and other grains consumption [11, 14]. In addition, the experimental literature on ZEA exposure indicates potential risk for detrimental health outcomes, yet there is limited data in humans. In this study, we aimed to characterize serum free and conjugated ZEA and its metabolites in adult women of various ages and determine whether circulating ZEA metabolites were associated with dietary intake of specific food groups that could contain mycoestrogens. Because both menopausal status and body weight affect estrogenic activity, it was hypothesized that these may also be predictors of ZEA metabolite levels.
2. Methods
2.1 Participants and Study Design
Fasting serum samples are from the Osteoporosis Weight Loss and Endocrine (OWLE; NIH-AG12161) study, aliquoted and frozen for storage at −70°C [15–17]. This cohort study was conducted in healthy, pre- and post-menopausal women (25–69 years of age). Healthy women were recruited at Rutgers University through local newspaper, electronic and radio station advertisements for clinical studies. Participants diagnosed with diseases (i.e. metabolic bone disease, hyperparathyroidism, untreated thyroid disease, significant immune, hepatic, or renal disease, kidney stone in the last 5 years, significant cardiac disease, active malignancy or cancer therapy within the past year) or taking menopausal hormone therapy were excluded, as reported previously [16, 18]. Premenopausal women who were not pregnant and postmenopausal women who had not menstruated for at least 2 years were included. Anthropometrics (height, weight, body mass index (BMI)) were measured by balance scale and stadiometer in the clinical laboratory. Certified phlebotomists performed the blood draws. Usual dietary intake was assessed by dietitians on the day of serum sample collections.
The protocols were approved by the Institutional Review Board of Rutgers University (New Brunswick, NJ) and all participants provided written informed consent prior to study procedures. In addition, all participants approved that their samples could be analyzed for questions in future studies.
2.2 Biomarker Analysis
Serum samples were transferred to the Chemical Core Analysis Facility of the Occupational and Environmental Health Sciences Institute (EOHSI), Rutgers University. ZEA and its metabolites were measured using LC-MS/MS technique. Total metabolite concentrations were determined with enzymatic deconjugation carried out by adding 10 µL β-glucuronidase from Helix pomatia (Type HP-2, Millipore Sigma, St. Louis, MO) to 0.5mL serum and 0.25 mL sodium acetate buffer (pH=4.65) and incubating overnight in a water bath at 37 °C. Free metabolites were measured by adding only the buffer to 0.5 ml serum followed by the incubation. After the two separate analysis for free and total metabolites, the conjugated were estimated from the total minus the free forms. Cleanup for both sample types was performed using a 1 ml ChemElut™ extraction cartridge (Agilent Technologies, Inc., Folsom, CA), eluting the analytes with three 2ml aliquots of methyl tert-butyl ether. The combined eluents were evaporated to dryness and redissolved in 35 µl of 2:1:1 water: methanol: acetonitrile. All analytes were separated and quantitated using a Thermo LTQ mass spectrometer interfaced to an LC system consisting of a Finnigan Surveyor Autosampler plus and a Finnigan Surveyor MS Pump plus. A Hypersil Gold C18, 50×2.1mm, 1.9µm (Thermo Scientific, San Jose, CA) was used for elution. The solvent gradient was as follows: initial 25% methanol, 50% water, 25% CAN, linear ramp to 35% methanol, 30% water, 35% ACN over 6 min, hold for 4 min, return to the starting composition in 0.01 min, equilibrate for 6 min. The flow rate was 0.2 ml/min. An injection volume of 20 µL was used. Retention times were: 2.30 min for taleranol, 2.46 min for β-zearalenol, 3.10 min for zeranol, 3.31 min for α-zearalenol, 4.04 min for zearalanone, and 4.17 min for ZEA. An atmospheric pressure chemical ionization source was used in negative mode to ionize ZEA and its metabolites before introduction into the mass spectrometer. The precursor ions for the MS method were: m/z 321(zeranol and taleranol), m/z 319 (α- and β-zearalenol, zearalanone) and m/z 317 (ZEA) and the quantitation ions were m/z 277 and m/z 303 (zeranol and taleranol), m/z 275 and m/z 301(α- and β-zearalenol, zearalanone), and m/z 273 and m/z 299 (ZEA). All quality standard control parameters were followed throughout the analysis (Supplemental Figure 2), including blanks and recoveries for each sample run. Analyte spikes were used for quality control and run with each batch of samples. Recoveries of ±20% were used to validate the quality of the run. The detection limit for the method was 0.07 ng/ml for all six analytes. When concentrations were below the limit of detection (<LOD) an estimated value was reported based on extrapolation of the curve to zero concentration. The inter-day assay variability (% RSD) was 4.5 for ZEA, 3.2 for α-zearalenol, 2.6 for β-zearalenol, 2.8 for zeranol, 3.0 for zearalanone, and 2.7 for taleranol. The intra-day assay variability (% RSD) was 4.0 for ZEA, 3.1 for α-zearalenol, 2.5 for β-zearalenol, 2.5 for zeranol, 2.8 for zearalanone, and 2.7 for taleranol. Levels of conjugated metabolites were estimated by subtracting the values for free metabolites from the values for total metabolites.
2.3 Food records
Dietary intake was determined using the average of three 24-hour dietary recalls, which were conducted by registered dietitians. Dietary intake of food groups was analyzed according to the American Diabetes Association (ADA) exchange groups including meat, grains/cereals, vegetables, fruits, dairy and we also assessed the number of eggs/day and included a bean/tofu category.
2.4 Statistical analyses
Descriptive statistics were used for participants’ demographics and to calculate mean, standard deviation, and minimum and maximum values for serum concentrations. One-way ANOVA and Bonferroni post-hoc was used to assess differences between meat groups or BMI category. Due to significant age differences between the different BMI groups, age was included as a covariate in the model (ANCOVA). Also, we tested for normality of the dependent variables using homogeneity of variance testing and as needed, log transformed the ZEA metabolites. Pearson correlation was performed to determine relationships between ZEA metabolites, food intake, weight and BMI. Since food was recorded in ounces as per the ADA exchange system, this unit was used to report values. Multiple regression analysis was used to assess the relative influence of independent variables (BMI, menopausal status and meat intake) on ZEA and ZEA metabolites. Statistical analyses were conducted using the SAS statistical package (SAS Institute, Cary, NC, USA; v 9.4). P value < 0.05 (2-sided) was considered statistically significant and data are presented as means ± SD.
3. Results
Selected characteristics for the women included in the analysis (n=48) are shown in Table 1. Participants were largely Caucasian (88%) and ranged in age from 25–69 years old, with a weight range of 42.7 – 123.2 kg and BMI range of 18.5–41.3 kg/m2. Women were categorized by BMI status, and 65% had a normal BMI and 35% were overweight or obese (BMI ≥ 25 kg/m2).
Table 1.
Characteristic of participants (n=48)
| Gender | |
| Female | 48 (100%) |
| Menopausal Status | |
| Premenopausal | 20 (42%) |
| Post | 28 (58%) |
| Age (years) | 52 ± 9 |
| Weight (kg) | 65.5 ± 15.6 |
| BMI (kg/m2) | 25.0 ± 5.4 |
| Normal weight | 22.0 ± 1.9 |
| Overweight/Obese | 30.5 ± 5.6 |
Data are Mean ± SD
3.1 Serum concentrations of ZEA and its metabolites
Serum concentrations of ZEA and its metabolites (ZEA metabolites) are shown in Table 2. The serum free ZEA metabolite concentration detection rate ranged from 6.3–85.4%. The conjugated concentration of individual metabolite detection ranged from 16.7–100%. The concentration of free ZEA metabolites in the serum was 0.069 ± 0.078 ng/mL and was 5.4% of the conjugated ZEA metabolites. Serum ZEA had the highest free and conjugated concentrations with a high rate of detection (Table 2).
Table 2.
| Metabolites | Free (ng/mL) | Conjugated (ng/mL) | ||||
|---|---|---|---|---|---|---|
| Detection | All values | Values > LOD | Detection | All Values | Values > LOD | |
| zearalenone (ZEA) | 85.4% | 0.026 ± 0.022 | 0.087 ± 0.020 | 100% | 0.603 ± 0.436 | 0.641± 0.419 |
| α-zearalenol | 6.3% | 0.001 ± 0.006 | none | 62.5% | 0.208 ± 0.321 | 0.444 ± 0.350 |
| β-zearalenol | 35.4% | 0.011 ± 0.021 | 0.089 ± 0.017 | 39.6% | 0.053 ± 0.161 | 0.231 ± 0.205 |
| zeranol | 16.7% | 0.005 ± 0.011 | None | 75.0% | 0.181 ± 0.218 | 0.300 ± 0.216 |
| zearalanone | 31.3% | 0.022 ± 0.043 | 0.102 ± 0.039 | 93.8% | 0.174 ± 0.128 | 0.203 ± 0.119 |
| taleranol | 8.3% | 0.001 ± 0.004 | None | 16.7% | 0.020 ± 0.060 | 0.176 ± 0.089 |
| Total ZEA metabolites | 97.9% | 0.066 ± 0.067 | 0.123 ± 0.077 | 100% | 1.264 ± 0.884 | 1.298 ± 0.867 |
Values are mean ± SD (n=48). Zero value is used for non-detectable samples. The total metabolites concentration is equal to the sum of zearalenone, zeranol, taleranol α-zearalenol, β-zearalenol, zearalanone concentrations. Detection indicates the percent of samples with values above zero.
The number of samples with values > LOD (0.07) for free and conjugated are as follows: ZEA (3, 45), zeranol (0, 28), taleranol (0, 5) α-zearalenol (0, 22), β-zearalenol (2, 16), zearalanone (9, 40), ZEA metabolites (11, 46).
3.2 Effect of food intake
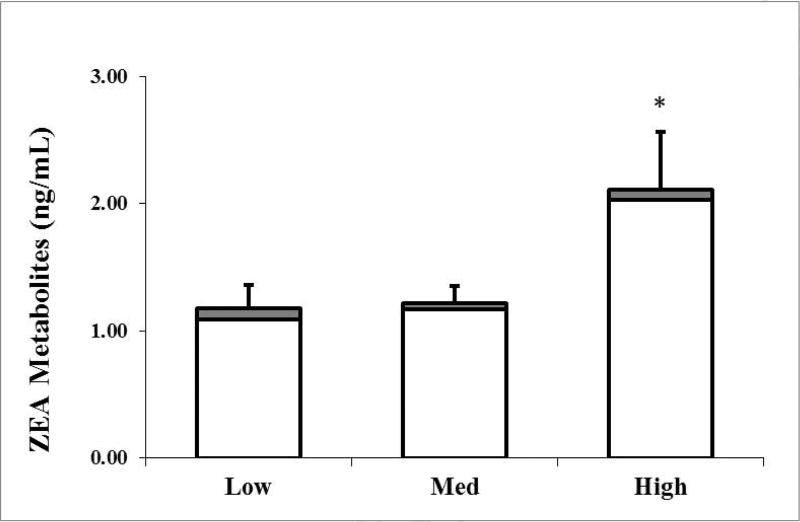
Several relevant food group intakes including meat, dairy, and cereal/grains were examined. Average meat intake was 5.7 ± 3.6 oz/d. In the low, medium, and high meat groups, intake was 2.8 ± 1.5, 6.7 ± 1.2, and 12.3 ± 2.8 oz/d, respectively. Meat intake was largely from poultry, and some pork. Beef intake was only 0.6 ± 1.4, 1.2 ± 1.8, and 4.6 ± 4.1 oz/d in the low, medium and high meat groups, respectively and did not differ significantly between groups. Mean BMI did not differ significantly between low, medium and high meat groups (25 ± 6 kg/m2; 24 ± 4 kg/m2 and 27 ± 8 kg/m2, respectively). There were higher serum conjugated ZEA metabolites found with high compared to the moderate and low meat intakes (p=0.05) (Figure 1). Conjugated serum β-zearalenol and ZEA was also greater in the high compared to lower meat groups (p < 0.05) (Supplemental Table 1). In addition, Pearson correlation indicated a positive relationship between meat intake and conjugated log ZEA and ZEA metabolites (r > 0.34, p = 0.02).
Figure 1. Free and conjugated serum ZEA Metabolites grouped by meat intake.
Conjugated:
 ; Free:
; Free:
 Participants were categorized by low (n=22; 0–4 oz /day or ~0–114 g/d], medium (n=19; 5–9 oz/day or 140–255 g/d), and high (n=7) meat intake (10–17 oz/d or 280–480 g/d). Values represent Mean ± SEM. Differs from low intake, *p = 0.05.
Participants were categorized by low (n=22; 0–4 oz /day or ~0–114 g/d], medium (n=19; 5–9 oz/day or 140–255 g/d), and high (n=7) meat intake (10–17 oz/d or 280–480 g/d). Values represent Mean ± SEM. Differs from low intake, *p = 0.05.
Intake of other foods included grains (starches including bread and cereal) that averaged 4.2 ± 2.6 servings per day, fruits and vegetables (4.5 ± 2.2/d), dairy intake (2.3 ± 1.4 servings/d) and there was 1 serving/d for nuts/bean/tofu and only 15% of the women reported consumption of 1–2 eggs/d. There were no significant differences in serum free or conjugated ZEA metabolites when participants were analyzed for any of the other food groups.
3.3 BMI categories
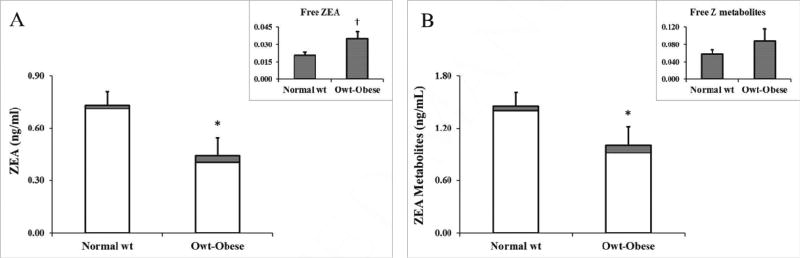
Women were grouped into BMI categories titled Normal Weight (Normal wt; BMI <25 kg/m2) and Overweight/Obese (Owt-Obese; BMI ≥ 25 kg/m2) and also characterized for ZEA metabolites. The mean BMI in the normal weight group was 22.0 ± 1.9 kg/m2 (n=31) and was 30.5 ± 5.6 kg/m2 in the Owt/Ob women (n=17). The normal weight women were found to have higher serum conjugated ZEA concentration (0.711 ± 0.412 ng/mL) than overweight/obese women (0.405 ± 0.403 ng/mL) (p<0.02) (Figure 2A). Serum conjugated ZEA metabolites showed a similar trend with higher concentrations in the normal than overweight/obese women (p < 0.07) (Figure 2B). Concentrations of free ZEA and ZEA metabolites were not significantly different between groups, but ZEA showed a trend to be higher in the overweight/obese than normal weight group (p = 0.09) (Figure 1A). Pearson correlation indicated that BMI showed a trend to be inversely associated with serum total ZEA (r = − 0.27, p < 0.07) but positively associated with both free ZEA and free ZEA metabolites (r > 0.37, p < 0.01).
Figure 2. Serum free and conjugated A) ZEA and B) ZEA metabolites grouped by BMI category.
Conjugated:
 ; Free:
; Free:

BMI categories: normal weight (wt) (BMI < 25; n=31) or overweight (owt) and obese (BMI > 25; n=17).
Values represent Mean ± SEM. Differs between groups, *p< 0.02, Ɨ p = 0.09.
Abbreviations: Body mass index (BMI), Zearalenone (ZEA), ZEA and its metabolites summed together (ZEA metabolites)
3.4 Pre- and Postmenopausal Status
Serum ZEA metabolite concentrations were also examined by menopausal status. Mean conjugated serum ZEA metabolites were higher in pre- than postmenopausal women (1.40 ± 0.645 vs 1.166 ± 1.007 ng/mL, p < 0.05). Also, conjugated serum zeranol was higher in pre-than post-menopausal women (0.337 ± 0.246 vs 0.070 ± 0.095, p < 0.005). No other free and conjugated ZEA metabolites were significantly different due to menopausal status.
3.5 Multiple Regression
Multiple regression analysis was performed to determine independent predictors of ZEA and ZEA metabolites. Findings (Table 3) showed that BMI predicted the serum free and conjugated ZEA and free ZEA metabolites (p < 0.05). Menopausal status only predicted serum free ZEA (p = 0.006). Meat intake predicted conjugated ZEA and ZEA metabolites (Table 3).
Table 3.
Multiple regression for serum ZEA and ZEA metabolites
| β coefficient | P value | Model R | β coefficient | P value | Model R | ||
|---|---|---|---|---|---|---|---|
| Serum | |||||||
|
| |||||||
| Free ZEA | Conjugated ZEA | ||||||
| BMI | 0.277 | 0.046 | 0.280 | −0.302 | 0.045 | 0.150 | |
| Menop. Status | 0.387 | 0.006 | −0.061 | 0.674 | |||
| Meat | −0.035 | 0.791 | 0.285 | 0.051 | |||
|
| |||||||
| Free ZEA metabolites | Conjugated ZEA metabolites | ||||||
| BMI | 0.379 | 0.023 | 0.139 | −0.142 | 0.054 | 0.159 | |
| Menop. Status | 0.012 | 0.940 | −0.107 | 0.191 | |||
| Meat | −0.071 | 0.633 | 0.389 | 0.024 | |||
Abbreviations: Menopausal (Menop) Zearalenone (ZEA), ZEA and its metabolites summed together (ZEA metabolites)
4. Discussion
Zearalenone and its metabolites can be considered mycoestrogens due to their similar structure in comparison to estradiol. In the USA and other countries, the major source of ZEA metabolites is through contamination of grains that are also fed to livestock [1, 2, 14, 19–22]. In addition, because ZEA metabolites are generally resistant to food manufacturing, they are present in a variety of foods. However, there are limited studies examining the concentration and detection of ZEA and its metabolites despite their potent estrogenic activity and potential effects on health outcomes. In the present study, a large percentage of serum samples showed detectable levels of ZEA and its metabolites in healthy women. In addition, there is an association between circulating conjugated ZEA metabolites with meat intake and BMI.
It was found that ZEA had the highest detection rate compared to the other metabolites. This is likely because ZEA is the parent compound that can be reduced to other metabolites, or conjugated with glucuronic acid or even sulfates. Bandera and colleagues investigated a population of young girls (n=163) and reported the free form of the urinary metabolites [8]. In this study of girls, there was a 78% detection rate for the total free urinary metabolites [8] whereas there was 98% detection in serum in this adult population. Methodologies used in our study and in this previously conducted study were similar. A case study was performed in a single healthy young adult male [9]. Interestingly, at baseline there was no detectable urinary ZEA, and after the high cereal diet, urinary total ZEA concentration was 0.39 ng/mL after one week. It is notable that exposure to ZEA or metabolites are negligible in some reports [10, 14, 23]. For example, in a Belgian population, there was minimal or no detection of urinary ZEA or its metabolites found in children or adults [23]. Less than 7% positive ZEA was detected in a pilot study in northern Nigeria population [14]. In addition, the urinary and serum ZEA concentration were also low in pregnant women [10]. In the current study, serum ZEA metabolites indicated more consistent detection, similar to findings in a previous study of older women [24]. It is likely that exposure differs across populations with different agriculture practices and types of foods preferred [25].
In this study, serum ZEA concentrations increased with meat intake. Participants with low meat intake, categorized as 0–4 oz/day, had significantly lower concentrations of total serum metabolites when compared to those with higher meat intakes, >10 oz/day. Compared to the 2015–2020 USA Dietary Recommended Intake for red meat and poultry at 26 oz/week (or approximately 4 oz/d), the low meat group in the current study was below this level of intake, compared to the higher meat intake groups that had intakes above these guidelines. To estimate whether ZEA was present in local foods, a preliminary study in our lab indicated detectable levels in beef and poultry (2–10 ug/kg) and in livestock feed (10–85 ug/kg), with very low levels (< 0.5 ug/kg) in grains for human consumption such as red quinoa, barley, sorghum, teff and spelt, and popcorn and corn flakes (< 0.6 ug/kg) and milk products (cow and goat < 0.01 ug/L) (unpublished data). Also, 20 other grains/cereals were tested with undetectable ZEA levels. The present investigation suggests that dietary intake from meat is a source of ZEA, possibly due to ZEA present in livestock feed. This differs from the Jersey Girl study [8] in the same geographic region as the current study, indicating beef intake and popcorn were associated with higher urinary ZEA, yet the middle-aged women reported here did not consume popcorn and only 35% of the women consumed beef. The findings in the current study attributing meat as a primary source of ZEA also differs from global studies suggesting that cereals and grains are a primary source of ZEA metabolites [1, 2, 14, 19, 20, 22, 25].
The established provisional maximal tolerable daily intake (PMTDI) for ZEA is 0.5 µg/kg/d [26–28]. The women in this analysis had a maximal meat intake/day of 17 oz (~0.5 kg meat/d) and the maximal ZEA detection level in local meat in this study was 10 µg/kg of meat. Using these values with an average body weight of 65.5 kg in the women, the calculated ZEA intake is 0.075 µg/kg daily intake, which is well below the 0.5 ug/kg/d PMTDI. However, it is more than double the estimated mean dietary intake of ZEA (0.03 µg/kg/d) in the USA [28]. It is possible that contamination in the food supply in the past decade has increased and/or that food sources of ZEA differ regionally, yet our data is limited to only a few select foods and hence there is a need for a more thorough assessment of ZEA levels in foods. In addition, the use of serum biomarkers of ZEA and metabolites may be useful in determining exposure or risk assessment, Furthermore, given that we found that circulating ZEA metabolites are altered with obesity, it is possible that while ZEA intake is below the PMTDI, it may have different health implications in vulnerable populations.
There is a difference in concentrations of ZEA and metabolites when analyzed by BMI category. With an increase in BMI, there was an increase in the concentration of serum free metabolites. This occurred despite a decline in ZEA and a trend to decrease total circulating ZEA metabolites. It is also known that females who are obese and overweight have higher levels of total and free serum estradiol (E2), compared to those with lean BMI, possibly due to higher activity of aromatase enzyme which is responsible for the biosynthesis of estrogen [29] [30]. In the case of ZEA metabolites, it is possible that adipose acts as a depot lowering ZEA metabolite concentrations [31, 32] in the obese or postmenopausal women with greater adiposity, and could explain the lower total circulating levels of ZEA and its metabolites in the current study. It is notable that ZEA has strong estrogenic activity as an agonist for estrogen receptor-α with possible antagonistic effects at low doses [33] and it possible that its effects differ under conditions of high or low estrogen status, such as menopause or pregnancy. In the current study, we found that free ZEA was lower in pre- than post-menopausal women indicating that circulating E2 may influence circulating levels. In addition, because free E2 binds directly with the estrogen receptor, and ZEA and its metabolites are more weakly bound to sex hormone binding globulin (SHBG) than E2, ZEA would be expected to replace some SHBG-bound E2, and thereby increase free-E2. It is possible that free and conjugated ZEA have different biological effects, and therefore measuring both ZEA and E2 and their free forms together in a future study might elucidate if there is a relationship affecting physiological outcomes.
The implications of low, but chronic intake, of this estrogen disruptor are not clear. High consumption of ZEA or zeranol has been associated with negative effects, as indicated by numerous studies in animals. Most studies show ZEA decreases follicle stimulating hormone after maturity, consistent with its estrogenic effects [34, 35]. An investigation of pregnant mice that were subcutaneously injected with ZEA indicated accelerated onset of puberty with a prolonged estrus cycle and accelerated mammary gland differentiation in the offspring [36]. Because ZEA has been shown to inhibit testosterone biosynthesis [37], the potential effects on growth and reproduction may not be limited to one sex. In other studies, a diet contaminated with ZEA decreased pro- and anti-inflammatory markers [38], adversely affected spleen function [39], and reproductive physiology [40] [41]. However, there is limited data suggesting that chronic or dietary exposure to ZEA metabolites has a significant effect on health outcomes in humans. For example, Pillay et al found ZEA metabolites in the plasma of both cervical and breast cancer patients and healthy controls, with no significant difference in levels between groups [24]. In Bandera et al. [8] ZEA exerted anti-estrogenic effects on body size and pubertal development. Together, these limited data indicate a need for replication studies in larger pools with more heterogeneous populations, designed to examine the effects of ZEA metabolites on health outcomes.
There are strengths and limitations of the present research. This study was a cross sectional analysis within a single geographic area in the NY metropolitan area and with relatively small samples. However, it is one of the larger studies examining circulating free and total ZEA metabolites [10, 25]. In addition, we did not measure serum E2 or sex hormone binding globulin, which would have assisted in better understanding the association between free and bound E2 and ZEA metabolites. The strength of these data is that women in this study represented a wide age and BMI range and dietary intake was carefully assessed with multiple recalls. Moreover, it is possible that the timing of meal intake [10] and sample collection would increase variability of circulating levels, but in this study, only fasting serum was used.
This study demonstrates detectable levels in nearly all women and that meat intake and obesity alters the levels, with potential implications for safety measures as it relates to health outcomes. Meat intake was a source of ZEA that may have been from contaminated corn/grains fed to livestock. Future research should address ZEA in populations with a wide age range, possibly examining those with high and low exposure and determining related health outcomes. Particularly important, studies should be conducted in susceptible populations such as young children or pregnant women.
Supplementary Material
The environmental estrogen, zearalenone (ZEA), is found in the food supply
There are limited studies examining ZEA and its metabolites, and in this study we found detectable serum levels in nearly all women.
Serum conjugated ZEA metabolites was higher in women who consumed more meat products.
Obesity lowered conjugated ZEA and total ZEA metabolites compared to normal weight, but not free concentrations.
Acknowledgments
Funding Sources
We thank Robert Zurfluh, RD, MS for the clinical efforts and recruitment, and the efforts of the technical staff in the LC/MS/MS laboratory at the Center for Environmental Exposures and Disease. This study was supported by NIH-NIEHS P30 ES005022, NIH-AG12161 and USDA-NIFA (NJAES − 0153866).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflict of interest to disclose.
References
- 1.Alshannaq A, Yu JH. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int J Environ Res Public Health. 2017;14 doi: 10.3390/ijerph14060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evaluation of certain mycotoxins in food. Fifty-sixth report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organ Tech Rep Ser. 2002;906:i–viii. 1–62. [PubMed] [Google Scholar]
- 3.Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleck SC, Hildebrand AA, Pfeiffer E, Metzler M. Catechol metabolites of zeranol and 17beta-estradiol: a comparative in vitro study on the induction of oxidative DNA damage and methylation by catechol-O-methyltransferase. Toxicol Lett. 2012;210:9–14. doi: 10.1016/j.toxlet.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Kuiper-Goodman T, Scott PM, Watanabe H. Risk assessment of the mycotoxin zearalenone. Regul Toxicol Pharmacol. 1987;7:253–306. doi: 10.1016/0273-2300(87)90037-7. [DOI] [PubMed] [Google Scholar]
- 6.Hueza IM, Raspantini PC, Raspantini LE, Latorre AO, Gorniak SL. Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins (Basel) 2014;6:1080–1095. doi: 10.3390/toxins6031080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Escriva L, Font G, Manyes L. In vivo toxicity studies of fusarium mycotoxins in the last decade: a review. Food Chem Toxicol. 2015;78:185–206. doi: 10.1016/j.fct.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Bandera EV, Chandran U, Buckley B, Lin Y, Isukapalli S, Marshall I, King M, Zarbl H. Urinary mycoestrogens, body size and breast development in New Jersey girls. Sci Total Environ. 2011;409:5221–5227. doi: 10.1016/j.scitotenv.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warth B, Sulyok M, Berthiller F, Schuhmacher R, Krska R. New insights into the human metabolism of the Fusarium mycotoxins deoxynivalenol and zearalenone. Toxicol Lett. 2013;220:88–94. doi: 10.1016/j.toxlet.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Fleck SC, Churchwell MI, Doerge DR, Teeguarden JG. Urine and serum biomonitoring of exposure to environmental estrogens II: Soy isoflavones and zearalenone in pregnant women. Food Chem Toxicol. 2016;95:19–27. doi: 10.1016/j.fct.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Shephard GS, Burger HM, Gambacorta L, Gong YY, Krska R, Rheeder JP, Solfrizzo M, Srey C, Sulyok M, Visconti A, et al. Multiple mycotoxin exposure determined by urinary biomarkers in rural subsistence farmers in the former Transkei, South Africa. Food Chem Toxicol. 2013;62:217–225. doi: 10.1016/j.fct.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 12.Wallin S, Gambacorta L, Kotova N, Lemming EW, Nalsen C, Solfrizzo M, Olsen M. Biomonitoring of concurrent mycotoxin exposure among adults in Sweden through urinary multi-biomarker analysis. Food Chem Toxicol. 2015;83:133–139. doi: 10.1016/j.fct.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Solfrizzo M, Gambacorta L, Visconti A. Assessment of multi-mycotoxin exposure in southern Italy by urinary multi-biomarker determination. Toxins (Basel) 2014;6:523–538. doi: 10.3390/toxins6020523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ezekiel CN, Warth B, Ogara IM, Abia WA, Ezekiel VC, Atehnkeng J, Sulyok M, Turner PC, Tayo GO, Krska R, Bandyopadhyay R. Mycotoxin exposure in rural residents in northern Nigeria: a pilot study using multi-urinary biomarkers. Environ Int. 2014;66:138–145. doi: 10.1016/j.envint.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Pop LC, Sukumar D, Tomaino K, Schlussel Y, Schneider SH, Gordon CL, Wang X, Shapses SA. Moderate weight loss in obese and overweight men preserves bone quality. Am J Clin Nutr. 2015;101:659–667. doi: 10.3945/ajcn.114.088534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sukumar D, Partridge NC, Wang X, Shapses SA. The high serum monocyte chemoattractant protein-1 in obesity is influenced by high parathyroid hormone and not adiposity. J Clin Endocrinol Metab. 2011;96:1852–1858. doi: 10.1210/jc.2010-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res. 2005;20:455–463. doi: 10.1359/JBMR.041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapses SA, Sukumar D, Schneider SH, Schlussel Y, Sherrell RM, Field MP, Ambia-Sobhan H. Vitamin D supplementation and calcium absorption during caloric restriction: a randomized double-blind trial. Am J Clin Nutr. 2013;97:637–645. doi: 10.3945/ajcn.112.044909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Boevre M, Jacxsens L, Lachat C, Eeckhout M, Di Mavungu JD, Audenaert K, Maene P, Haesaert G, Kolsteren P, De Meulenaer B, De Saeger S. Human exposure to mycotoxins and their masked forms through cereal-based foods in Belgium. Toxicol Lett. 2013;218:281–292. doi: 10.1016/j.toxlet.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Chain EPoCitF. Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA Journal. 2011;9 [Google Scholar]
- 21.Evaluation of certain food additives and contaminants. World Health Organ Tech Rep Ser. 2000;896:1–128. [PubMed] [Google Scholar]
- 22.Codex Committee on Food Additives and Contaminants (CCFAC) Joint FAO/WHO Expert Committee on Food Additives: Position Paper on Zearalenone. In: Commission CA, editor. Rome, Italy: 2000. [Google Scholar]
- 23.Heyndrickx E, Sioen I, Huybrechts B, Callebaut A, De Henauw S, De Saeger S. Human biomonitoring of multiple mycotoxins in the Belgian population: Results of the BIOMYCO study. Environ Int. 2015;84:82–89. doi: 10.1016/j.envint.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Pillay D, Chuturgoon AA, Nevines E, Manickum T, Deppe W, Dutton MF. The quantitative analysis of zearalenone and its derivatives in plasma of patients with breast and cervical cancer. Clin Chem Lab Med. 2002;40:946–951. doi: 10.1515/CCLM.2002.166. [DOI] [PubMed] [Google Scholar]
- 25.Mally A, Solfrizzo M, Degen GH. Biomonitoring of the mycotoxin Zearalenone: current state-of-the art and application to human exposure assessment. Arch Toxicol. 2016;90:1281–1292. doi: 10.1007/s00204-016-1704-0. [DOI] [PubMed] [Google Scholar]
- 26.JECFA. Evaluation of certain food additives and contaminants. Fifty-third Report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organ Tech Rep Ser. 2010 [Google Scholar]
- 27.Massart F, Saggese G. Oestrogenic mycotoxin exposures and precocious pubertal development. Int J Androl. 2010;33:369–376. doi: 10.1111/j.1365-2605.2009.01009.x. [DOI] [PubMed] [Google Scholar]
- 28.Zinedine A, Soriano JM, Molto JC, Manes J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: an oestrogenic mycotoxin. Food Chem Toxicol. 2007;45:1–18. doi: 10.1016/j.fct.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 29.Karim R, Mack WJ, Hodis HN, Roy S, Stanczyk FZ. Influence of age and obesity on serum estradiol, estrone, and sex hormone binding globulin concentrations following oral estrogen administration in postmenopausal women. J Clin Endocrinol Metab. 2009;94:4136–4143. doi: 10.1210/jc.2009-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyvernitakis I, Knoll D, Struck M, Hars O, Bauer T, Hadji P. Impact of BMI on serum estradiol and bone turnover markers in postmenopausal women with hormone-sensitive early breast cancer treated with anastrozole. J Cancer Res Clin Oncol. 2014;140:159–166. doi: 10.1007/s00432-013-1557-3. [DOI] [PubMed] [Google Scholar]
- 31.Shin BS, Hong SH, Bulitta JB, Hwang SW, Kim HJ, Lee JB, Yang SD, Kim JE, Yoon HS, Kim DJ, Yoo SD. Disposition, oral bioavailability, and tissue distribution of zearalenone in rats at various dose levels. J Toxicol Environ Health A. 2009;72:1406–1411. doi: 10.1080/15287390903212774. [DOI] [PubMed] [Google Scholar]
- 32.Escriva L, Font G, Manyes L, Berrada H. Studies on the Presence of Mycotoxins in Biological Samples: An Overview. Toxins (Basel) 2017;9 doi: 10.3390/toxins9080251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Burns KA, Arao Y, Luh CJ, Korach KS. Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor alpha and beta in vitro. Environ Health Perspect. 2012;120:1029–1035. doi: 10.1289/ehp.1104689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Dobaib SN, Mehaia MA, Khalil MH. Effect of feeding discarded dates on milk yield and composition of Aradi goats. Small Ruminant Research. 2009;81:167–170. [Google Scholar]
- 35.Triusu RJ, Cowie CC, Harris MI. Hormone replacement therapy and glucose metabolism. Obstet Gynecol. 2000;96:665–670. doi: 10.1016/s0029-7844(00)00980-7. [DOI] [PubMed] [Google Scholar]
- 36.Nikaido Y, Yoshizawa K, Danbara N, Tsujita-Kyutoku M, Yuri T, Uehara N, Tsubura A. Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring. Reprod Toxicol. 2004;18:803–811. doi: 10.1016/j.reprotox.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Liu Q, Wang Y, Gu J, Yuan Y, Liu X, Zheng W, Huang Q, Liu Z, Bian J. Zearalenone inhibits testosterone biosynthesis in mouse Leydig cells via the crosstalk of estrogen receptor signaling and orphan nuclear receptor Nur77 expression. Toxicol In Vitro. 2014;28:647–656. doi: 10.1016/j.tiv.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Pistol GC, Gras MA, Marin DE, Israel-Roming F, Stancu M, Taranu I. Natural feed contaminant zearalenone decreases the expressions of important pro- and anti-inflammatory mediators and mitogen-activated protein kinase/NF-kappaB signalling molecules in pigs. Br J Nutr. 2014;111:452–464. doi: 10.1017/S0007114513002675. [DOI] [PubMed] [Google Scholar]
- 39.Tiemann U, Brüssow KP, Pöhland R, Schneider F, Jonas L, Dänicke S. Effects of diets with cereal grains contaminated by graded levels of two Fusarium toxins on selected immunological and histological measurements in the spleen of gilts. Journal of Animal Science. 2006;84:236–245. doi: 10.2527/2006.841236x. [DOI] [PubMed] [Google Scholar]
- 40.Alm H, Brussow KP, Torner H, Vanselow J, Tomek W, Danicke S, Tiemann U. Influence of Fusarium-toxin contaminated feed on initial quality and meiotic competence of gilt oocytes. Reprod Toxicol. 2006;22:44–50. doi: 10.1016/j.reprotox.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Doll S, Gericke S, Danicke S, Raila J, Ueberschar KH, Valenta H, Schnurrbusch U, Schweigert FJ, Flachowsky G. The efficacy of a modified aluminosilicate as a detoxifying agent in Fusarium toxin contaminated maize containing diets for piglets. J Anim Physiol Anim Nutr (Berl) 2005;89:342–358. doi: 10.1111/j.1439-0396.2005.00527.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.