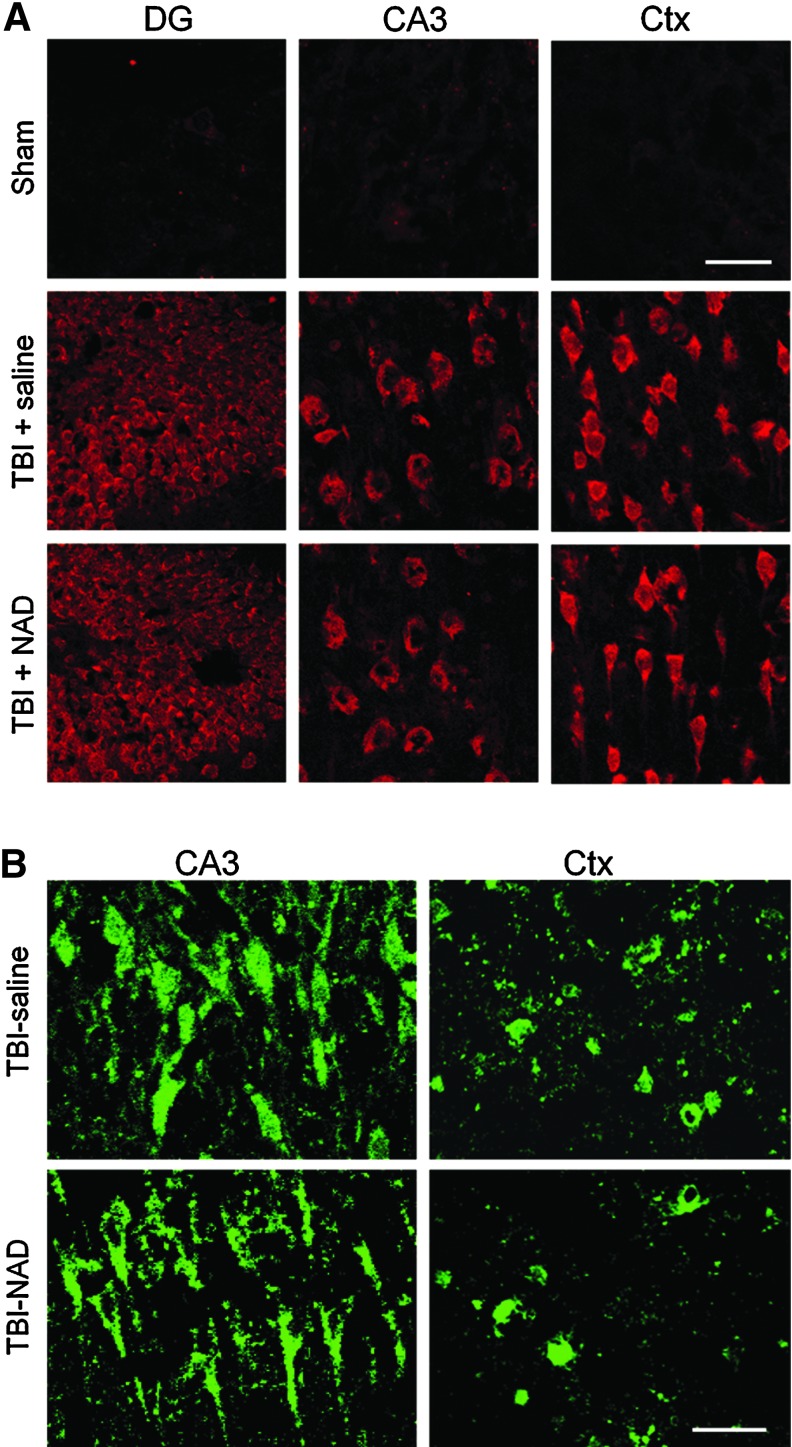
FIG. 5.
Traumatic brain injury (TBI)-induced reactive oxygen species (ROS) production and poly(ADP-ribose) polymerase-1 (PARP-1) activation are not prevented by intranasal nicotinamide adenine dinucleotide (NAD+) administration. (A) TBI-induced ROS production is not prevented by intranasal NAD+ administration. Neuronal superoxide production in the hippocampus and cortex was detected by dihydroethidium (dHEt) fluorescence staining 3 h after TBI. In sham-operated animals (Sham), Et fluorescent signal is negligible. TBI substantially increased ethidium (Et) intensity in the hippocampal CA3 and dentate gyrus neurons, and cortical (Ctx) neurons. Intranasal treatment with NAD+ caused no difference in Et fluorescence intensity after TBI (n=5 for each group; scale bar=30 μm). (B) TBI-induced poly(ADP-ribose) (PAR) accumulation is not prevented by intranasal administration of NAD+ in the hippocampus and cortex. Neuronal poly (ADP-ribose) (PAR) accumulation in the hippocampus and cortex was detected by immunohistochemistry 3 h after TBI. TBI substantially increased PAR fluorescence intensity in hippocampal CA3 and in cortical neurons. However, NAD+ treatment showed no difference in intensity of PAR fluorescence after TBI (n=3 for each group; scale bar=30 μm). Color image is available online at www.liebertonline.com/neu