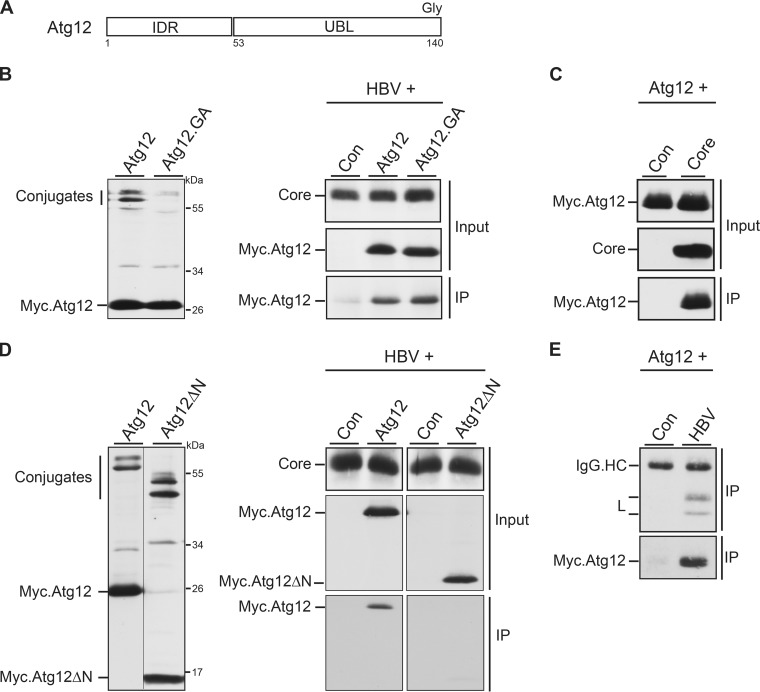
FIG 5.
Atg12 associates with HBV core via its IDR domain and is incorporated into HBV particles. (A) Schematic depiction of Atg12 with its N-terminal IDR and C-terminal UBL domains. The terminal glycine (Gly) residue, required for Atg5 conjugation, is indicated. (B) Expression profile of Myc-tagged Atg12 and its conjugation-defective Atg12.GA mutant in transiently transfected HuH-7 cells. Cell lysates were probed by Myc-specific WB. Unconjugated and conjugated Atg12 forms are indicated (left). For co-IP, the HBV replicon was cotransfected with the control, Atg12, or Atg12.GA expression construct at a 3:1 DNA ratio (right). Synthesis of core, ATG12, and ATG12.GA is shown by WB of lysates with anticore and anti-Myc antibodies (Input). For IP, lysates were incubated with anticore antibodies followed by Myc-specific WB. (C) IP analysis of cells cotransfected with Myc-tagged Atg12 and a control or core-expressing plasmid was done as described in the legend to panel B. (D) Expression analysis of Myc-tagged Atg12 and the Atg12ΔN mutant, lacking the IDR domain, in transfected HuH-7 cells. Unconjugated and conjugated Atg12 forms are depicted. The co-IP analysis was done as described in the legend to panel B. (E) Cellular supernatants of HuH-7 cells, cotransfected with the Myc-tagged Atg12 construct plus a control or pHBV, were subjected to an L-specific IP to precipitate extracellular virions. Immunoprecipitates were then immunoblotted with anti-L (top) or anti-Myc (bottom) antibodies. IgG.HC, unspecifically stained immunoglobulin G heavy chains.