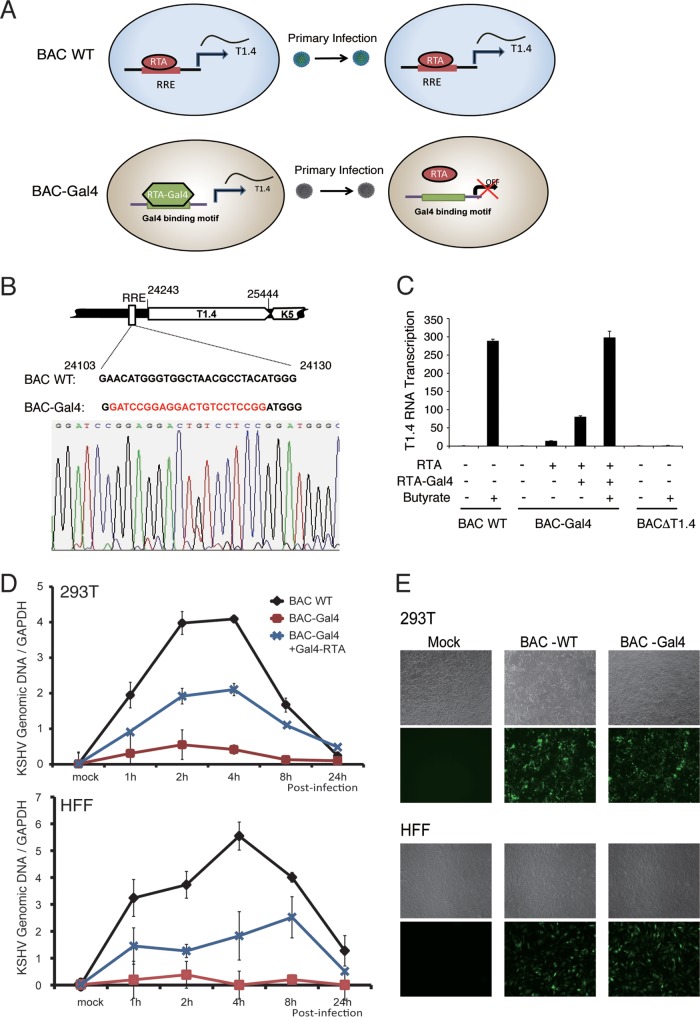
FIG 10.
T1.4 RNA is essential for KSHV DNA replication following de novo infection. (A) Schematic presentation of the experimental design for conditional expression of T1.4 to demonstrate its role in viral DNA replication using RTA-Gal4 mutant virus. In WT virus, RTA binds the RRE to induce the expression of T1.4 RNA and lytic replication, followed by virion release. During de novo infection, RTA binds to the RRE to induce T1.4 RNA for the initiation of DNA replication. In BAC-Gal4 mutant virus, the RRE in the T1.4 promoter has been replaced with a Gal4 binding sequence. T1.4 expression is induced by ectopic expression of RTA-Gal4 fusion protein, and BAC-Gal4 mutant virus is produced. When BAC-Gal4 virions infect fresh cells, RTA is unable to bind to the Gal4 motif in the T1.4 promoter, so no T1.4 is transcribed. (B) Construction of BAC-Gal4, where the RRE sequence in ori-Lyt has been replaced with a Gal4 binding motif. The mutant virus was confirmed by sequencing. (C) Validation of the expression of T1.4 RNA in BAC16 (BAC WT) and BAC-Gal4. (D) BAC-Gal4 does not support viral DNA replication following de novo infection. 293T cells and HFF were infected by BAC16 (WT) or BAC-Gal4 at an MOI of 50 (viral genomic DNA equivalents). To induce the expression of T1.4 in BAC-Gal4 virus, 293T cells and HFF were transfected with Gal4-RTA expression vector. Twenty-four hours posttransfection, the cells were infected with BAC16 and BAC-Gal4 viruses. The viral DNA content was determined at different time points postinfection by qPCR analysis. The viral genomic DNA copy number was normalized by GAPDH. The error bars indicate SD. (E) The infectivity of BAC-WT and BAC-Gal4 viruses was examined 24 h postinfection by GFP expression (green fluorescence) under a fluorescence microscope at ×5 magnification.