Figure 1.
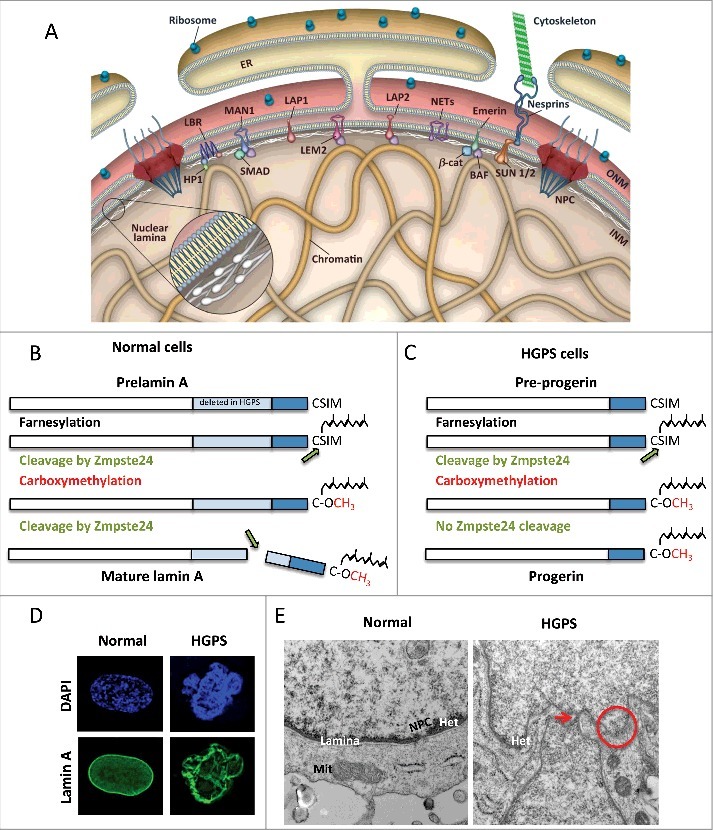
A-type lamins structure and post-translational processing are important for nuclear stability. (A) Schematic representation of the nuclear envelope. The nuclear lamina is a network of intermediate filaments underneath the inner nuclear membrane (INM) that associates with chromatin and INM proteins. Adopted from Dobrzynska et al., Nucleus 2016. (B) Lamin-A is synthesized as a pre-lamin-A precursor that undergoes processing of its C-terminus to render mature lamin-A. The -CAAX motif undergoes farnesylation, cleavage of the last three amino acids, followed by carboxyl methylation of the terminal cysteine. This farnesylated form of the protein has high affinity for the inner nuclear membrane. A second cleavage by the metalloprotease Zmpste24 removes 15 amino acids, including the farnesylated and methylated cysteine, rendering mature lamin-A. (C) HGPS patient-derived cells carry mutations in the LMNA gene that cause aberrant splicing of the gene and deletion of 50 residues that include the Zmpste24 cleavage site. This permanently farnesylated and methylated truncated form of lamin-A is known as progerin. (D) HGPS patient-derived fibroblasts cells exhibit profound nuclear morphological abnormalities, as shown by immunofluorescence with lamin-A antibody. (E) Normal and HGPS fibroblasts were processed for electron microscopy to detect structural defects in nuclei. In normal fibroblasts, note how the nuclear lamina is located underneath the nuclear membrane, and associated with heterochromatin domains (dark staining). A nuclear pore complex is also evident. In HGPS cells, nuclear morphological abnormalities such as protrusions and invaginations (arrow), and sites of nuclear membrane discontinuity (circle) are evident.
