Figure 2.
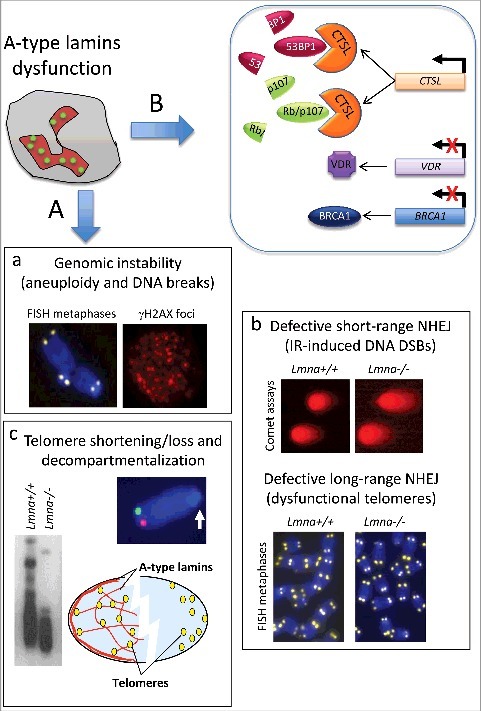
A-type lamins as caretakers of the genome. (A) Hallmarks of genomic instability that have been reported in A-type lamins-deficient cells include: (a) Aneuploidy and increased frequency of chromosome/chromatid breaks (as shown by telomere-FISH on metaphase spreads), which result in accumulation of DNA damage (γH2AX foci by immunofluorescence); (b) Defects in short-range and long-range NHEJ mechanism of DNA DSB repair, as shown by neutral comet assays performed after ionizing radiation-induced DNA DSBs (short-range), and by FISH on metaphases induced to undergo NHEJ of dysfunctional telomeres (long-range); (c) Telomere shortening (as assessed by Telomere Restriction Fragment analysis), or complete loss, and decompartmentalization of telomeres in the 3D nuclear space (as shown by 3D-telomere-FISH). (B) At a molecular level, reduced expression of A-type lamins causes transcriptional upregulation of CTSL gene, and increased global cathepsin L (CTSL) activity. CTSL is responsible for the degradation of 53BP1, and the Rb family members pRb and p107, which are key factors in DNA repair and cell cycle regulation, respectively. The decrease in 53BP1 underlies defects in short-range and long-range NHEJ. In addition, lamins deficiency elicits downregulation of vitamin D receptor (VDR) and BRCA1 gene expression, events that impair HDR repair. Other factors are likely to contribute to genomic instability in different laminopathies such as accumulation of XPA at DSBs, deficiency in DNA-PK holoenzyme function, increased generation of ROS, and epigenetic alterations (not shown in the model).
