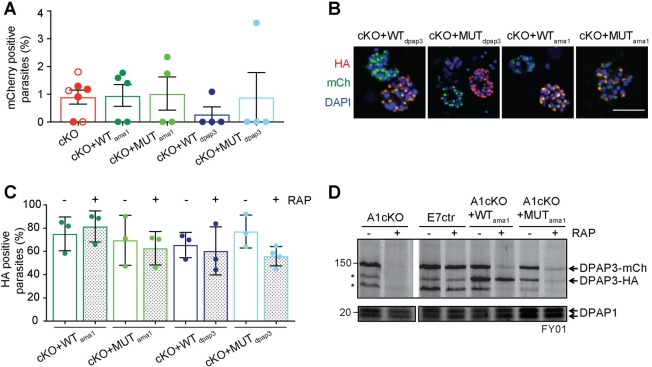
Fig 5. Generation of complementation lines.
(A) Quantification of excision efficiency for DPAP3cKO and complementation lines. Schizonts collected after DMSO or RAP treatment were stained with anti-mCherry and anti-SUB1 antibodies. SUB1 staining was used as a marker of schizont maturity. The amount of mCherry positive schizonts was quantified in relation to the total amount of mature schizonts. In DMSO treated parasites, all mature schizonts were mCherry positive (100%). The amount of non-excised parasites after RAP treatment was <5% in all biological replicates for all the cKO and complementation lines tested. Each circle corresponds to a different biological replicate (>100 schizonts were analyzed per biological isolate). Filled circles correspond to F8cKO and its complementation lines, and empty ones to the A1cKO line. (B) IFA analysis of the complementation lines showing colocalization of chromosomal DPAP3-mCh and episomal DPAP3-HA expressed under the dpap3 or ama1 promoters. Mature schizonts from the F8cKO+WTdpap3, F8cKO+MUTdpap3, F8cKO+WTama1, and F8cKO+MUTama1 were fixed and stained with anti-HA (red) and anti-mCherry (green). DNA was stained with DAPI (blue); scale bar: 5μm. Single coloured images are shown in S6C Fig. (C) Quantification of the amount of DPAP3-HA positive schizonts in the complementation lines. Schizonts were fixed at 48 h.p.i. and stained with anti-HA and anti-SUB1 antibodies. Only 60–80% of mature schizonts show positive HA staining with no difference between DMSO and RAP treated parasites. More than 100 schizonts per biological replicate were analyzed. (D) FY01 labelling of cKO and complementation lines. After DMSO or RAP treatment at ring stage, C2-arrested schizonts were collected, lysed, and labelled with FY01. Labelling of chromosomal DPAP3-mCh is clearly visible as a band around 150kDa along with some post-lysis degradation products indicated by asterisks. The loss of this 150kDa upon RAP treatment is observed in all lines except the E7ctr. Episomal WT DPAP3-HA is labelled by FY01 independently of RAP treatment and co-migrates with one of the degradation products of DPAP3-mCh at 125kDa. No labelling of MUT DPAP3-HA was observed. DPAP1 labelling by FY01 is shown as a loading control.