Figure 2.
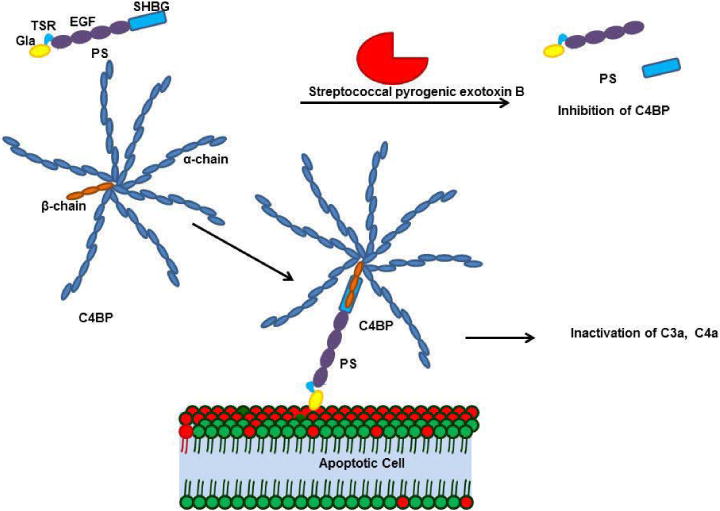
The PS-C4BP complex is essential for inhibiting inflammation. C4BP is a multimer formed by seven alpha chains and one beta chain crosslinked by cysteine disulfide bonds; the C4BP beta chain binds to the sex hormone binding globulin domain (SHBG) of PS. PS-C4BP further binds to the phosphatidylserine in the apoptotic cell membrane and inactivates complements such as C3a and C4a. Streptococcal pyrogenic exotoxin B (SPEB) inactivates the PS-C4BP complex during streptococcal infection. SPEB cleaves PS within the SHBG domain, preventing the PS-C4BP complex from binding the membrane.
