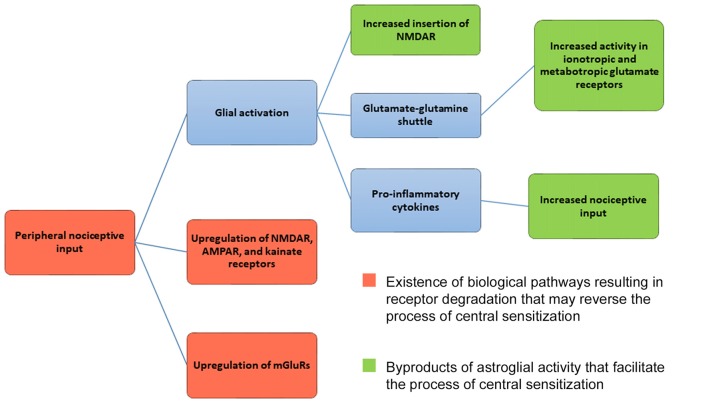
Figure 1.
Mechanism for central sensitization. Central sensitization (central nervous system hypersensitivity) is initiated from the upregulation of ionotropic glutamate receptors (NMDAR, AMPAR, kainite receptors) and metabotropic glutamate receptors (mGluRs) in the presence of peripheral nociceptive input. As a result, neurons in the dorsal horn of the spinal cord and central nervous system respond to nociceptive input at lower thresholds, with new enlarged receptor fields, and undergo increased rates of spontaneous firing. Glial activation can further maintain the mechanisms underlying central sensitization by increasing NMDAR and AMPAR insertion in postsynaptic membranes. Glial cells release pro-inflammatory cytokines, serving as further nociceptive input. Astroglial cells also help to maintain glutamate levels via the glutamate-glutamine shuttle, which can influence both ionotropic and mGluR activity. As shown in red, ionotropic, metabotropic receptors and nociceptors are capable of being degraded. Given that degradative pathways exist, the process of central sensitization can be reversed. Activity by astroglial cells, however, may mitigate the effects of receptor degradation by upregulating and facilitating the process of central sensitization. Given that central sensitization does not reverse itself with time, it seems that astroglial activity overpowers the existence of degradative receptor pathways.