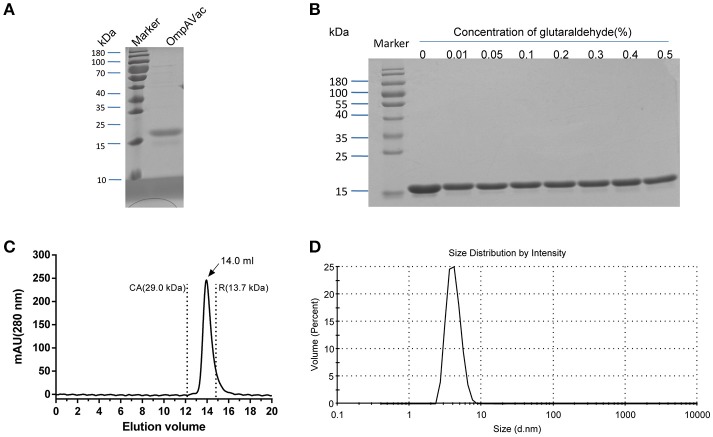
Figure 2.
Characterization of purified OmpAVac. (A) SDS-PAGE analysis of OmpAVac. The purity of OmpAVac was ~93.2%, as determined based on the density of the corresponding band in an SDS-PAGE gel. (B) Cross-linking assay of OmpAVac. The concentrations of glutaraldehyde in lanes 1–8 were 0, 0.01, 0.05, 0.1, 0.2, 0.3, 0.4, and 0.5%, respectively. No oligomers or aggregates were observed. (C) Chromatography analysis of OmpAVac. OmpAVac produces a symmetrical peak at 14.0 mL, and the elution volumes of the protein standards CA (carbonic anhydrase) and R (ribonuclease A) were 12.2 and 13.7 mL, respectively. (D) Dynamic light-scattering analysis of OmpAVac resulted in a symmetrical peak with a diameter of 3.8 nm.