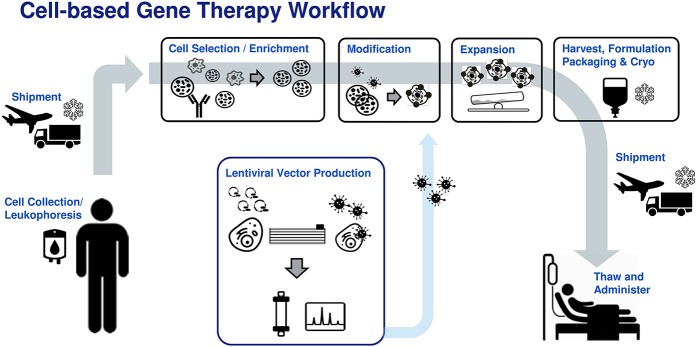
Figure 1.
Generic patient specific adoptive immunotherapy workflow. Donor leukophoresis sample undergoes selection to enrich for the cell of interest (e.g., T-cells, NK cells). Previously manufactured viral vector is added to genetically modify the immune cells, conferring new cancer recognition receptors. The modified cells are then expanded to therapeutically relevant numbers, followed by washing, formulation, bagging, and cryostorage into an infusible patient dose. The cell product is shipped to the clinical site where it is thawed and administered to the patient.