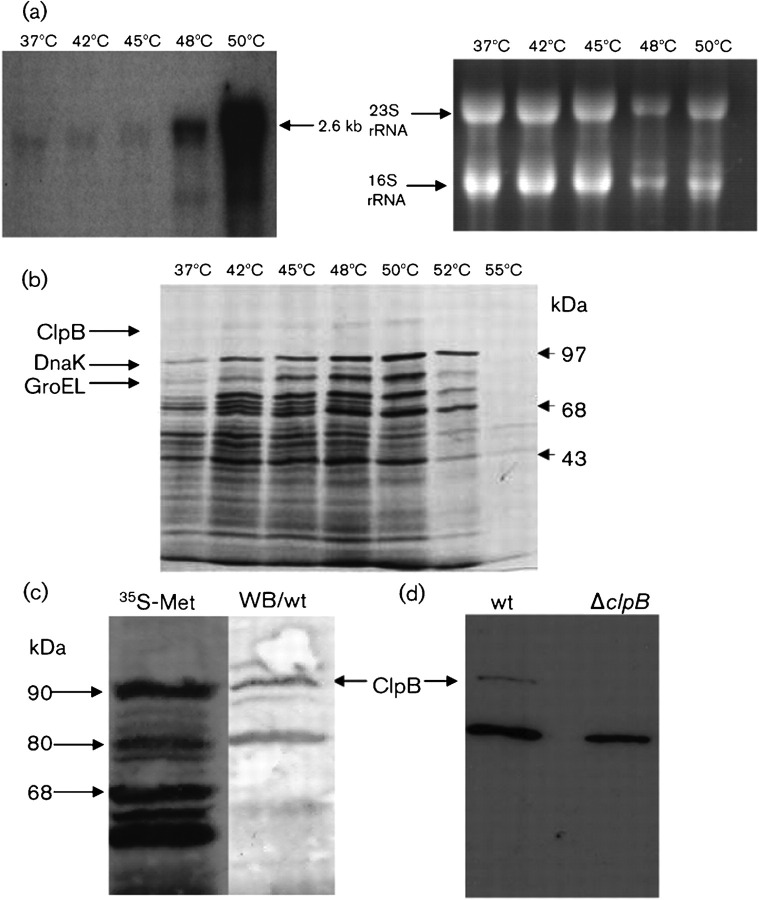
Fig. 3.
Induction of clpB mRNA and ClpB in response to heat shock. (a) Northern blot analysis of the E. faecalis clpB gene. In order to stimulate the production of the heat-shock mRNA, the cells were either cultivated at 37 °C (control cells) or stressed by upshift to 42, 45, 48 or 50 °C for 10 min. Total RNA was separated under denaturing conditions, transferred to a nitrocellulose membrane, and hybridized to a clpB-specific probe (left). A parallel gel was stained with ethidium bromide to show equal loading of the slots (right). (b) Influence of supra-optimum temperatures on protein synthesis. Wild-type cells were either incubated at 37 °C (control) or treated at 42, 45, 48, 50, 52 or 55 °C in the presence of [35S]methionine. Labelled proteins were separated by SDS-PAGE and analysed by autoradiography. The ClpB protein is indicated. DnaK and GroEL, which were previously identified, are also indicated (Laport et al., 2001). (c) Identification of the ClpB protein. Wild-type (wt) cells were subjected to heat shock at 45 °C in the presence of [35S]methionine. Labelled proteins were separated by SDS-PAGE and analysed by either autoradiogram (35S-Met) or Western blotting using polyclonal antibodies raised against Synechococcus spp. ClpB diluted 1 : 1000. (d) Western blotting of wild-type (wt) or ΔclpB cells subjected to heat shock at 45 °C showing the cross-reaction of the ClpB polyclonal antibody.