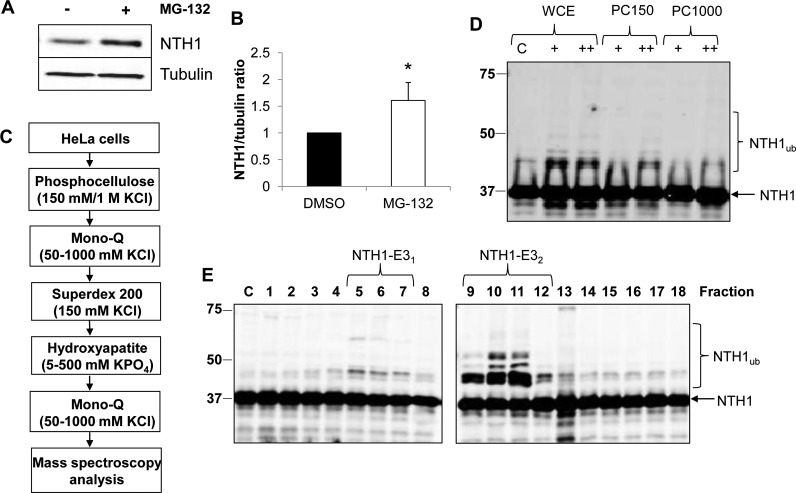
FIG 1.
Purification of E3 ubiquitin ligase for NTH1. (A) HCT116 cells grown in 10-cm dishes were treated with the proteasomal inhibitor MG-132 (10 μM) for 8 h. Whole-cell extracts were prepared and analyzed by 10% SDS-PAGE and immunoblotting with the indicated antibodies. (B) NTH1 protein levels in the absence and presence of MG-132 were quantified from the results of at least three independent experiments. Shown is the mean NTH1/tubulin ratio with standard deviation normalized to the DMSO-treated control, which was set to 1.0. *, P < 0.05 as analyzed by a two-sample t test. (C) Scheme for purification of the E3 ubiquitin ligase for NTH1 from HeLa cell extracts. (D) In vitro ubiquitylation of His-tagged NTH1 by HeLa whole-cell extract (WCE) and fractions obtained from phosphocellulose chromatography following low-salt elution (PC150) and high-salt elution (PC1000). + and ++, 2.5-μg and 5-μg fractions, respectively. (E) In vitro ubiquitylation of His-tagged NTH1 using fractions from the first ion exchange (Mono Q) chromatography. Ubiquitylation of His-tagged NTH1 (6 pmol) was performed in the presence of E1 activating enzyme (0.7 pmol), ubiquitin (0.6 nmol) (Ub), and all E2 conjugating enzymes (2.5 pmol) and analyzed by 10% SDS-PAGE and immunoblotting using NTH1 antibodies. Lane C, control reactions in the absence of any fraction. Molecular mass markers (in kilodaltons) are indicated on the left, and the positions of unmodified and ubiquitylated NTH1 (NTH1ub) are shown.