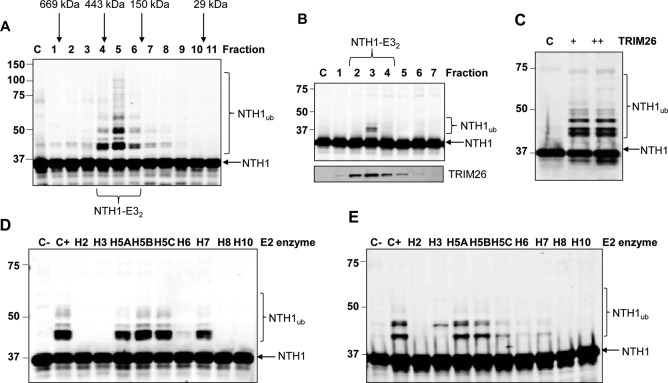
FIG 2.
Purification and identification of TRIM26 as the major E3 ubiquitin ligase for NTH1. (A) In vitro ubiquitylation of His-tagged NTH1 by fractions obtained from size exclusion (Superdex 200) chromatography. Shown above the blot are the positions of elution of known protein molecular mass standards. (B) In vitro ubiquitylation of His-tagged NTH1 by fractions obtained from the final ion exchange (Mono Q) chromatography. Shown below the blot is the alignment of active fractions with TRIM26 protein, as detected by immunoblotting. (C) In vitro ubiquitylation of His-tagged NTH1 by His-tagged TRIM26. Lane C, control reaction in the absence of any fraction. + and ++, 19 pmol and 26 pmol TRIM26, respectively. (D and E) Comparison of in vitro ubiquitylation of NTH1 by an active fraction purified from HeLa whole-cell extracts (D) or His-tagged TRIM26 (19 pmol) (E) in the presence of individual E2 conjugating enzymes. Control reactions in the absence (C−) or presence (C+) of all E2 enzymes are shown. Unless otherwise indicated, in all the experiments, in vitro ubiquitylation of His-tagged NTH1 (6 pmol) was performed in the presence of E1 activating enzyme (0.7 pmol), ubiquitin (0.6 nmol), and E2 conjugating enzymes (2.5 pmol) and analyzed by 10% SDS-PAGE and immunoblotting using NTH1 antibodies. Molecular mass markers (in kilodaltons) are indicated on the left, and the positions of unmodified and ubiquitylated NTH1 (NTH1ub) are shown.