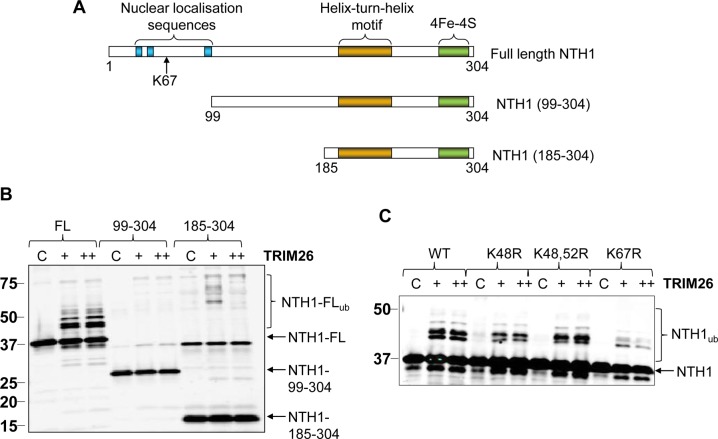
FIG 4.
Identification of sites of ubiquitylation within NTH1 by TRIM26. (A) Schematic showing the protein domains within the full-length NTH1 protein and two N-terminal truncations of NTH1 (99 to 304 and 185 to 304). (B) In vitro ubiquitylation of His-tagged full-length NTH1 and truncations of NTH1 by His-tagged TRIM26. (C) In vitro ubiquitylation of His-tagged NTH1 mutants by His-tagged TRIM26. In all the experiments, in vitro ubiquitylation of His-tagged NTH1 (6 pmol) was performed in the presence of E1 activating enzyme (0.7 pmol), H5A E2 conjugating enzyme (2.5 pmol), and ubiquitin (0.6 nmol). + and ++, 19 pmol and 26 pmol TRIM26, respectively. Control reactions in the absence of any His-tagged TRIM26 (lanes C) are shown. All the reactions were analyzed by 10% SDS-PAGE and immunoblotting using NTH1 antibodies. WT, wild type. Molecular mass markers (in kilodaltons) are indicated on the left, and the positions of unmodified and ubiquitylated NTH1 (NTH1ub) are shown.