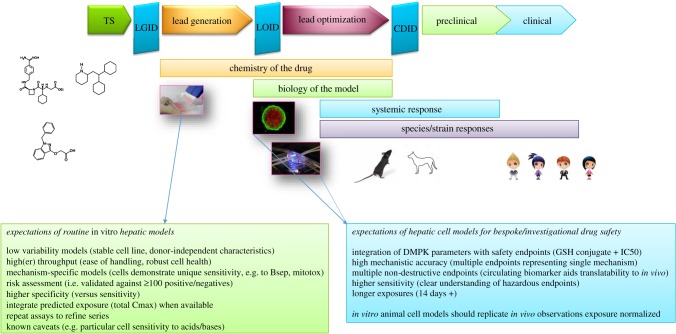
Figure 2.
Positioning and performance expectations of hepatic safety assays. Earlier routine risk assessment assays have generally been validated with +100 compounds, which either cause or are clean for hepatotoxicity. These assays are designed to remove the worst offending chemistry, cover the main mechanisms responsible for hepatotoxicity and have defined sensitivity and specificity characteristics. At later stages of the pipeline, there is potential for refining risk assessment to patients by engineering susceptibility characteristics into hepatocytes such that functionality is altered, which may affect sensitivity to certain compounds. TS, target selection; LOID, lead optimization investment decision; CDID, candidate drug investment decision; DMPK, drug metabolism pharmacokinetics.