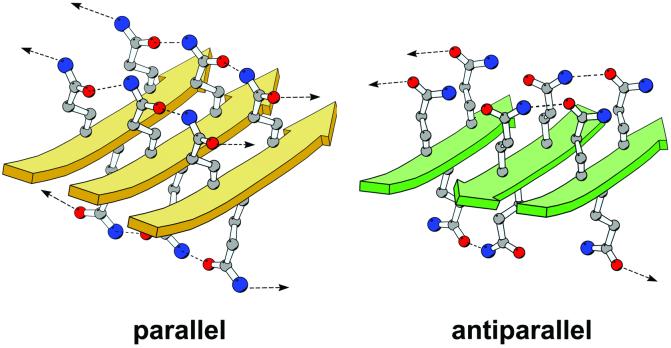
Figure 6.
Molecular models of homopolymeric glutamine peptides forming either parallel β-sheets (gold) or antiparallel β-sheets (green). Models containing four 11-residue strands were built in QUANTA (Accelrys, San Diego) and energy-minimized with CHARMM (Harvard Univ., Cambridge, MA; ref. 37); only portions of the models are shown for the sake of clarity. The parallel model was constructed starting from a canonical parallel β-sheet architecture followed by a search for optimal side-chain torsion angles. The antiparallel model is based upon figure 1 in ref. 16. The strands of the β-sheet are represented by solid arrows, and the glutamine side chains are shown in a ball-and-stick rendering, with carbon atoms colored in gray, nitrogen atoms in blue, and oxygen atoms in red. Dashed lines represent hydrogen bonds between side chains. Arrows represent hydrogen bonds to adjacent strands that are not included in the figure. The figure was made with the program MOLSCRIPT (38).