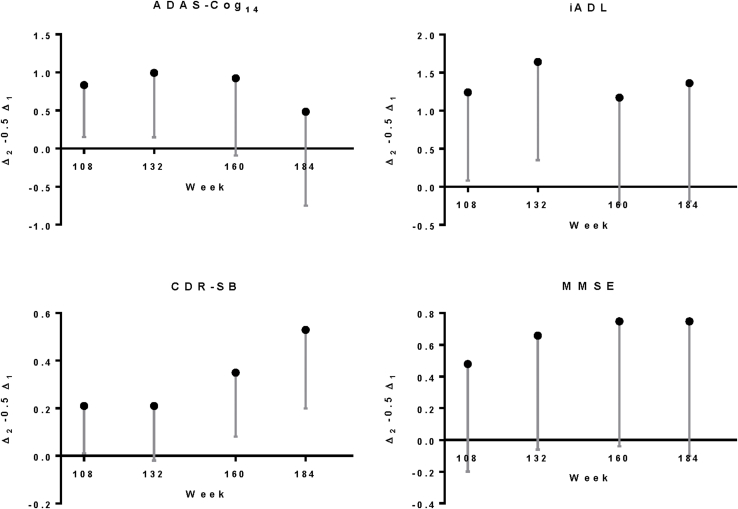
Fig. S1.
Noninferiority statistics for early-start compared with delayed-start treatment groups for ADAS-Cog14, ADCS-iADL, CDR-SB, and MMSE among patients with mild AD in the solanezumab Expedition program through 3.5 years. Point estimates represent Δ2–0.5Δ1, and error bars represent the lower 90% confidence limit. Abbreviations: ADAS-Cog14, 14-item Alzheimer's disease assessment scale–cognitive subscale; ADCS-iADL, Alzheimer's Disease Cooperative Study activities of daily living inventory, instrumental items; CDR-SB, clinical dementia rating scale–sum of boxes; MMSE, mini-mental status examination; AD, Alzheimer's disease.