Abstract
Inhaled general anesthetics are used commonly in adults and children, and a growing body of literature from animals and humans suggests that exposure to anesthesia at an early age can impact brain development. While the origin of these effects is not well understood, it is known that anesthesia can disrupt oxygen regulation in the brain, which is critically important for maintaining healthy brain function. Here we investigated how anesthesia affected brain tissue oxygen regulation in neonatal rabbits by comparing brain tissue oxygen and single unit activity in the awake and anesthetized states. We tested two common general anesthetics, isoflurane and sevoflurane, delivered in both air and 80% oxygen. Our findings show that general anesthetics can greatly increase brain tissue PO2 in neonates, especially when combined with supplemental oxygen. Although isoflurane and sevoflurane belong to the same class of anesthetics, notable differences were observed in their effects upon neuronal activity and spontaneous respiration. Our findings point to the need to consider the potential effects of hyperoxia when supplemental oxygen is utilized, particularly in children and neonates.
Keywords: Electrophysiology, Isoflurane, Rabbit, Sevoflurane, Single Unit
Graphical abstract
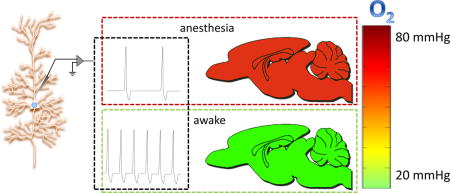
1. Introduction
Inhaled general anesthetics are used commonly in the course of surgery, imaging and other medical procedures in adults and children, yet their impact upon brain function is not entirely understood. It is known that these drugs suppress neuronal activity by acting primarily upon GABA receptors (Jia et al., 2008) and cause decreased firing frequency in a majority of neurons. As they also produce depression of vascular and respiratory centers (Bosch et al., 2017), it is important to consider how these anesthetics may affect oxygen regulation within the brain.
Normal brain function depends upon maintaining the oxygen partial pressure (PO2) in brain tissue within a relatively narrow range that is sufficiently high to prevent hypoxia and low enough to minimize generation of toxic oxygen species. The brain achieves this balance by ensuring stable blood flow via cerebrovascular autoregulation (Dagal and Lam, 2009), which regulates the response of cerebral arteries to fluctuations in arterial pressure, ensuring sufficient oxygen for basal neuronal activity. In addition, brain PO2 is regulated indirectly by carotid bodies and the respiratory center, which adjust breathing rate in response to arterial PO2 (Prabhakar and Semenza, 2015). More focal changes in neuronal activity are supported by the hemodynamic response (Buxton et al., 2004), which dilates local blood vessels in order to increase blood flow to active brain regions.
This system potentially can be disrupted not only by pathologies such as cerebrovascular diseases (Goadsby, 2013), but also by drugs, including general anesthetics, which decrease neuronal firing and depress respiration, altering oxygen consumption and delivery in brain tissue. Moreover, it has been found that at higher concentrations general anesthetics can directly affect vessels, causing vasodilation (Matta et al., 1999) and thus the increased delivery of oxygen to brain tissue. These opposing effects create a complex picture of the influence of general anesthetics upon oxygen regulation in the brain. However, our previous findings (Aksenov et al., 2012; Aksenov et al., 2015) suggest that the decrease in neuronal activity during anesthesia is large enough to produce an overall increase in brain tissue oxygen.
Changes in tissue PO2 induced by anesthesia are of particular concern for the developing brain. A number of studies have linked anesthesia exposure in young children with the development of a variety of pathologies later in life (Flick et al., 2011; Lee et al., 2015). Both human and animal studies have indicated (Crosby and Davis, 2013; Lee et al., 2015; Olsen and Brambrink, 2013; Sun, 2010; Taylor, 2009) that exposure to anesthesia, especially at an early age, can affect a variety of aspects of neuronal development, leading to deficits in learning and memory. Although the origin of these complications is not yet known, changes in PO2 could play a role. General anesthesia often is delivered in hyperoxic mixtures (typically 60%, 80% or 100% O2) (Edmark et al., 2003) in order to avoid hypoxia, yet hyperoxia resulting from brain tissue oxygen dysregulation can potentially lead to damage as well (Scheuer et al., 2017).
The goal of this work is to evaluate the effects of general anesthesia on the regulation of tissue PO2 in neonatal rabbits. Based on our previous findings regarding the effects of anesthesia on neuronal activity and tissue PO2 in adult rabbits, we hypothesized that brain tissue oxygen regulation would be significantly altered in neonates under general anesthesia during spontaneous respiration. In order to assess the impact of anesthesia on brain oxygen regulation, we used chronically implanted electrodes to measure PO2 and single unit activity in the somatosensory cortex in neonatal rabbits. By using the same electrodes to record PO2 and single unit firing rate, we were able to examine these changes at precisely the same location. Two anesthetics were chosen: sevoflurane, which is the most commonly used general anesthetic in children and adults, and isoflurane, which is often used in the developing world, as well as for research studies in animal models. Anesthesia delivery was tested in air as well as in combination with 80% O2. Our results indicate that each drug affected to a different degree neuronal firing and intracortical tissue PO2, as well as respiration, and that the tissue PO2 changes during hyperoxia were significantly greater when anesthesia was delivered with a higher-than-air oxygen concentration.
2. Material and Methods
2.1. Animal preparation
Five Dutch-belted rabbits (9-12 days-old) were used in accordance with the National Institutes of Health guidelines and protocols approved by the NorthShore University HealthSystem Research Institute Institutional Animal Care and Use Committee. The rabbit kits were born in a nest box containing shredded aspen bedding which was prepared in advance. The newborn rabbit kits were housed and nursed with the dam and began to open their eyes at 9-11 days.
At the age of 6 days animals were implanted with electrodes. For this procedure, animals were anesthetized with a mixture of ketamine (60 mg/kg) and xylazine (10 mg/kg). The recording assembly consisted of a silica tube (Polymicro Technologies, Phoenix, AZ) containing a bundle of four 25μm diameter gold-silver alloy microwires with formvar insulation (California Fine Wire, Grover Beach, CA) that were gold-plated at the tip. These electrodes terminated at different levels within a distance of 100 μm. The microwires were connected to a small 6-pin connector that was embedded in dental acrylic. A 100 μm silver chloride wire was placed between the skull and dura mater to serve as the reference electrode. During implantation surgery, lambda was positioned at the level of bregma and the stereotaxic coordinates were as follows: anterior-posterior was 2 mm ventral to bregma, medial-lateral was 2.5 mm from midline, and dorsal-ventral was under visual control. After implantation, the electrode assembly was cemented to the skull using dental acrylic and 3 nylon support screws. The average surgery duration was 1 hour. After completion of experiments, kits were euthanized on day 12th.
2.2. Experimental design
All experiments were performed beginning 3 days after surgery. The kits were restrained by means of a cloth sleeve. A recirculating warm water heating pad (T-Pump, Gaymar Industries Inc, Orchard Park, NY, USA) was used to maintain temperature. Respiration was measured using a pressure pad/respiration transducer (TSD110) (Biopac Systems, Inc, Goleta, CA, USA). PO2 and single unit activity were recorded in separate experiments first in the awake state for 5-6 min. Kits then were anesthetized with either isoflurane (Piramal Enterprises Limited, Kohir Mandal, Andhra Pradesh, India) or sevoflurane (AbbVie Inc., North Chicago, IL, USA) at 1 MAC via a custom mask using calibrated vaporizers (Drager Vapor 19.1). The level of 1 MAC was determined by toe and tail pinch test and was on average 2% isoflurane and 4% sevoflurane for this group of animals. Anesthesia was delivered for 6 min in air, then for 6 min in 80% oxygen, and finally in air again for 6 min. Anesthesia was then stopped and recordings continued during the period of recovery for 8-9 minutes, after which the signals typically were lost due to excessive movements of the kits. Each kit was exposed to isoflurane and sevoflurane on different days using this paradigm.
Additional experiments were performed in each kit to measure the PO2 response to 80% oxygen in the awake state. PO2 was recorded for 1 min during air breathing. Oxygen concentration was then increased to 80% and PO2 was recorded for 2 min. The inspired gas was then changed back to air, and PO2 was recorded for an additional 2 min.
After each session rabbits were returned to the dam. Best efforts were made to prevent rejection of the rabbits by the dam (e.g., kits were wrapped in the nesting materials to maintain normal smell). None of the kits were rejected by the dam.
2.3. PO2 and electrophysiological recording
The same electrodes were used in separate experiments for electrophysiology and PO2 recording. The PO2 electrodes were polarized at −0.7 volts, and connected to an ammeter (Keithley 614) to record a current that was typically 3 to 10 nA during air breathing. The current was converted to voltage, notch and low-pass filtered (30 or 50 Hz), and amplified. As the electrodes were chronically implanted, PO2 was calculated from current based on the initial calibration. In addition to quantitative analysis the direct fast Fourier transform was used to analyze the power spectrum of PO2 data. After completion of experiments the same rabbits were used for single unit recording before and during isoflurane or sevoflurane delivery on different days. The multiple signals from the microwires were fed through a miniature preamplifier to a multichannel differential amplifier system (Neuralynx Inc, Bozeman, Montana, USA). The signals were amplified, band-pass–filtered (300Hz to 3 kHz), and digitized (32 kHz/channel) using a Neuralynx data acquisition system. Unit discrimination was performed offline using threshold detection followed by a cluster analysis of individual action potential wave shapes using Neuralynx analysis software. For each phase of exposure, the data were analyzed using the last minute of recording prior to the change in anesthesia or inspired oxygen concentration.
Electrode locations were confirmed by MRI using a 9.4T imaging spectrometer (BioSpec 94/30USR, Bruker Biospin MRI GmbH) operating at 1H frequency of 400MHz. The spectrometer was equipped with an actively-shielded gradient coil (BFG-240-150-S-7, Research Resonance, Inc., Billerica, MA, USA). A single-turn, 40mm-diameter circular RF surface coil was used for both transmission and reception. Anatomical images were acquired from subjects following euthanasia using a multi-slice gradient echo pulse sequence with a TR of 1.5 s, a TE of 11 ms, a 21.7mm×21.7mm FOV, and a matrix size of 128×128, corresponding to an in-plane resolution of 170 μm × 170 μm.
2.4. Statistical analysis
Paired t-test was used to determine the significance of the difference between the changes in PO2, SU and respiration signals before, during and after anesthesia. One-way ANOVA (Statistica, StatSoft, Tulsa, OK) was used to compare differences in PO2, single unit, and respiration signals between isoflurane and sevoflurane. Power analysis using Statistica software (Root Mean Square Standardized Effect (RMSSE)) confirmed that N=5 was sufficient to reach power >0.85 using two-side F test at alpha level of .05 to evaluate differences in PO2, single units and respiration. Pearson correlation analysis was used to calculate the strength and direction of a linear relationship between the changes in single unit activity before and during anesthesia. Fisher z-transformation was used to establish the significance of the correlation coefficients. The data are presented as mean +/− SEM unless otherwise specified.
3. Results
3.1. Effect of anesthetics on brain tissue PO2
Each anesthetic produced changes in tissue PO2, which were exacerbated when delivered with 80% oxygen (Figure 1). When delivered in air, isoflurane (Fig. 1a) increased brain tissue PO2 (p<0.018), whereas sevoflurane did not (Fig. 1b). This increase in PO2 occurred in all five isoflurane-anesthetized subjects. In contrast, only 1 out of 5 sevoflurane-anesthetized subjects showed an increase in tissue PO2 during air-breathing.
Figure 1.
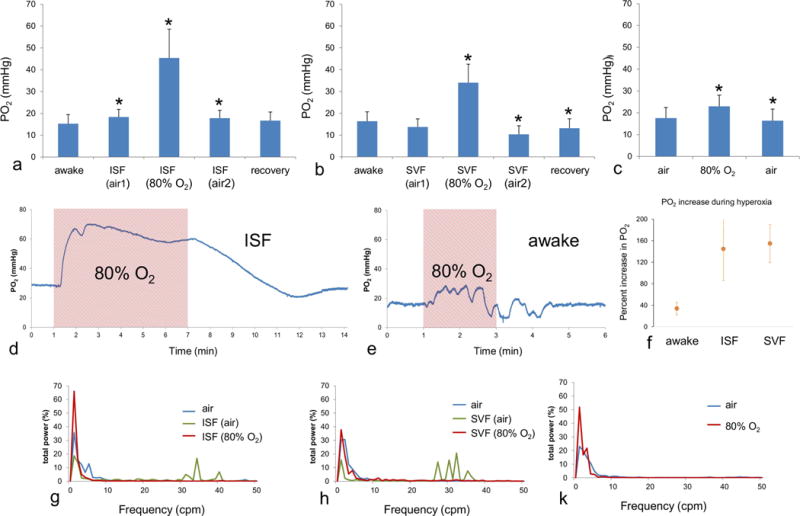
Effect of anesthesia on brain tissue PO2 in neonatal rabbits Isoflurane (a) produced a significant change in tissue PO2 during delivery in both air and 80% oxygen in neonates. The second ISF (air) bar represents the time period after 80% O2, and the recovery bar represents the time after anesthesia ended. Asterisks indicate a significant change (p<0.05) relative to the previous stage of exposure. Sevoflurane in air (b) did not produce a significant PO2 change relative to the awake state, but a large increase occurred with 80% oxygen. For both anesthetics PO2 recovered after the end of anesthesia. Respiration of 80% oxygen in the awake state (c) produced a significant increase in PO2 that was much smaller than during anesthesia and returned to the original level after air breathing resumed. Examples are shown of the PO2 response to 80% oxygen (shaded area) during isoflurane anesthesia (d) and in the awake state (e). A large increase occurred during 80% oxygen with sevoflurane, and the signal returned to baseline within several minutes when air was restored as the inspired gas. The PO2 response during the awake state, in comparison, was considerably smaller. (f) The mean percentage change in PO2 during 80% oxygen inspiration relative to air is shown for subjects during the awake state as well as anesthesia with each drug. The power spectra, averaged across animals, of tissue PO2 signals is shown for isoflurane (g) and sevoflurane (h) anesthesia, as well as for air and 80% oxygen in the awake state (i). Higher frequency peaks, likely corresponding to respiration, appeared in the spectra during anesthesia with air.
Brain tissue PO2 increased significantly for both isoflurane (p<0.04) and sevoflurane (p<0.009) when delivered in 80% O2, compared to anesthesia in air. For isoflurane the mean PO2 during anesthesia in 80% O2 was 45.4±13.2 mmHg, and for sevoflurane it was 34±8.5 mmHg. All subjects in both anesthetic groups exhibited an increase in tissue PO2 when breathing 80% O2
For both isoflurane (p<0.04) and sevoflurane (p<0.006), tissue PO2 decreased after reducing the inspired oxygen level from 80% to air, and then returned nearly to the awake level following the end of anesthesia.
For comparison we also tested the effect of inspired 80% O2 on tissue PO2 in fully awake neonates (Fig. 1c). Exposure to 80% O2 increased the tissue PO2 to 23±5.1 mmHg, from t 17.6 +/−4.8 mmHg under air (p< 0.014). This increase varied considerably across subjects, ranging from 2% to 60%, but was smaller in each animal than the change during anesthesia. Examples of the PO2 response to 80% oxygen are shown during anesthesia (isoflurane, Fig. 1d) and the awake state (Fig. 1e). The percentage change in mean PO2 during 80% oxygen respiration was dramatically larger during anesthesia than in the awake state (Fig. 1f).
Brain PO2 exhibits spontaneous fluctuations (Aalkjaer et al., 2011; Hudetz et al., 1998) whose origin is not clear. This effect is seen clearly in the trace of Fig. 1e, The fluctuations were evaluated using power spectrum analysis. The power spectrum (Fig. 1g,h,k) of PO2 in the awake state was dominated by low frequencies of 0-7 cycles per minute (cpm). For both isoflurane and sevoflurane a larger fraction of the power occurred at higher frequencies during anesthesia in air (30-41 cpm for isoflurane and 26-37 cpm for sevoflurane). These higher frequencies corresponded to respiratory frequency, and thus more of the fluctuations were the result of respiration as opposed to other spontaneous processes. This frequency distribution did not occur with anesthesia during 80% O2.
3.2. Effect of anesthetics on respiration
Isoflurane in air (Fig. 2a) slightly decreased the respiratory rate to 88% of the awake level (p< 0.028). Delivery of isoflurane in 80% O2 further decreased the respiratory rate to 83% of the initial level (p< 0.004). The depression of respiration under sevoflurane (Fig. 2b) was more severe. Sevoflurane in air decreased the respiratory rate to 80% of the awake level (p< 0.0003). When delivered in 80% O2 the respiratory rate under sevoflurane decreased further to 70% of the initial level (p< 0.015). Compared to isoflurane, the effect of sevoflurane on respiratory rate was significantly greater for both air (p< 0.019, F=8.7) and 80% O2 (p< 0.0003, F=37.03). For both anesthetics respiration fully returned to normal 10 min after the end of anesthesia.
Figure 2.
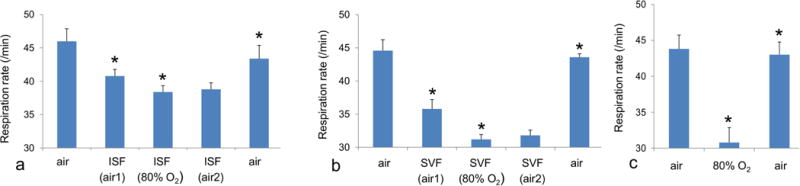
Effect of anesthesia on respiratory rate in neonatal rabbits during anesthesia in air and 80% oxygen, as well as during recovery for isoflurane (a) and sevoflurane (b). Respiratory rate also is shown before, during and after respiration of 80% oxygen without anesthesia (c). Asterisks indicate a significant change (p<0.05) relative to the previous stage. For both drugs respiration decreases significantly during anesthesia in air and 80% oxygen. Following the termination of anesthesia, respiration returns to the original rate.
We also examined the effect of 80% O2 on respiratory rate in fully awake neonates (Fig. 2c). During 80% O2 the respiratory rate decreased to 71.4±7.1% of the initial level (p< 0.024). The decrease of respiration in awake subjects with 80% O2 was significantly greater than the decrease under isoflurane in 80% O2 (p<0.0098, F=11.32). There was no significant difference between the respiratory rates in awake vs. sevoflurane-anesthetized neonates under 80% O2.
3.3. Effect of anesthetics on single unit responses
Each anesthetic decreased single unit activity to a different degree. Figure 3 shows the temporal behavior of a selected subset of recorded cells, which illustrates the range of recovery rates and suppression of activity that was observed for both anesthetics. The mean values (Figure 4) were calculated at the plateau level using the last minute of recording prior to the change in anesthesia or inspired oxygen concentration. As shown in Fig. 4a, isoflurane in air greatly decreased mean single unit activity (N=14) to 15% of the awake level (p<0.00009). Increasing the oxygen concentration to 80% did not produce any further change in single unit activity, relative to delivery in air. Single unit activity recovered to 41% of the awake level 9 minutes after the end of anesthetic delivery, which represented a significant change (p<0.001).
Figure 3.
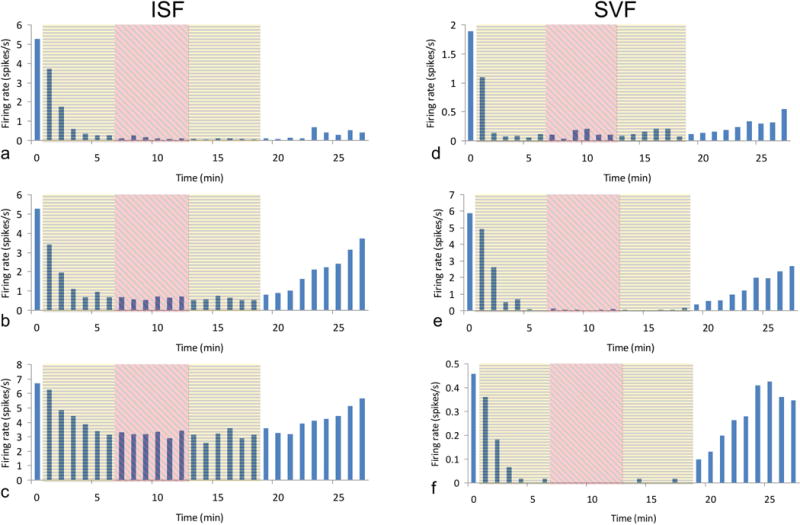
Temporal behavior of selected single units. Examples are shown of individual single units at each stage of exposure for isoflurane (a-c) and sevoflurane (d-f) anesthesia. In all cases neuronal activity decreased to a plateau level during anesthesia in air (yellow shading) within approximately 5 min of the onset of delivery, and no further change was observed during anesthesia in 80% oxygen (red shading). For both anesthetics a considerable variation was seen in the recovery time. For isoflurane some cells were greatly suppressed, whereas others showed much less change in firing rate. The decrease in neuronal activity was deeper and more consistent for sevoflurane, with periods of complete suppression which last 1 minute or longer.
Figure 4.
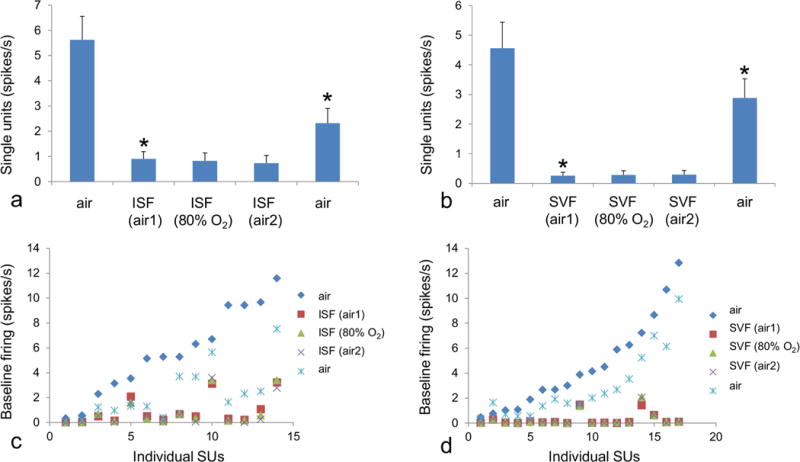
Effect of anesthesia on single unit responses in somatosensory cortex of neonatal rabbits. Both isoflurane (a) and sevoflurane (b) anesthesia in air greatly decreased mean single unit firing from the awake state. No further change occurred with 80% oxygen. Asterisks indicate a significant change (p<0.05) relative to the previous stage. Following anesthesia, single unit activity recovered, though not to the initial level within the time recorded. The decrease in neuronal activity was greater under sevoflurane than isoflurane. Individual single units, sorted by increasing initial firing frequency, are also shown for isoflurane (c) and sevoflurane (d) at each stage of anesthesia exposure. A more consistent decrease was recorded across all single units under sevoflurane as compared to isoflurane.
Sevoflurane in air (Fig. 4b) had an even greater effect on single unit activity than isoflurane. During sevoflurane, mean single unit activity decreased to 5.7% of the awake level (p<0.00013). As was the case for isoflurane, increasing the inspired oxygen concentration to 80% resulted in no further change in single unit activity. Overall, during sevoflurane anesthesia three out of 17 recorded single units showed no firing for 4 or more minutes. Nine minutes after the end of anesthesia single unit activity recovered to 63% of the awake level, which is indicative of a quicker rate of recovery for sevoflurane as compared to isoflurane (p<0.0009, F=16.4), (figure 4c,d).
Additional differences in the effects of isoflurane vs. sevoflurane could be observed in the pattern of firing of the individual single units (Figs. 4c,d). Whereas virtually all single units were suppressed under sevoflurane, the effect of isoflurane was more variable and only a subset of the single units were fully suppressed, and none of the single units ceased firing for 1 min or more as was the case during sevoflurane. Overall, sevoflurane was characterized by a greater and more uniform recovery than isoflurane. There was no significant correlation between the firing rate of cells in the awake state and the amount of depression induced by isoflurane or sevoflurane anesthesia.
4. Discussion
The primary effect of general anesthesia is to induce in the brain a widespread decrease of neuronal activity. In this state neurons require less oxygen, and thus it might be expected that the level of oxygen in brain tissue either would be down-regulated to match this lower neuronal demand or would remain at a constant level if brain tissue oxygen is regulated by a more active mechanism. However, our results show that the opposite occurs.
Even when delivered in air, isoflurane increased brain tissue PO2. This effect is likely the result not only of decreased neuronal firing and correspondingly lower oxygen consumption, but also anesthesia-induced vasodilation which occurs in both adults and children (Brenet et al., 1998; Luginbuehl et al., 2003; Lyons et al., 2016) and would increase the delivery of oxygen to brain tissue. Vasodilatation can occur during anesthesia due to direct agonistic action upon the GABA receptors present on vessels (Matta et al., 1999). Moreover, hypercapnia (Eastwood et al., 2016; Kaiser et al., 2005) resulting from decreased respiration potentially could contribute to vasodilation as well. Sevoflurane delivered in air did not produce a significant increase in tissue PO2, but sevoflurane anesthesia also suppressed respiration to a much greater extent than isoflurane, which would result in lower delivery of oxygen to the brain and thus a smaller change in tissue PO2 despite potential contributions from direct GABA-ergic vasodilatory effects and hypercapnia.
The most compelling evidence of an anesthetic-induced disturbance in matching flow to demand comes from the results obtained during 80% inspired oxygen. Both isoflurane and sevoflurane greatly increased brain tissue PO2 relative to the awake state, where PO2 still can be regulated in hyperoxia. In some subjects tissue PO2 was up to 5 times greater than in the awake state. In general cerebral oxygenation is an important consideration in neonatal medicine. A number of clinical reports indicate that a fraction of inspired oxygen (FiO2) ranging from 0.3 to 1.0 may be used in pediatric practice (for example, (Ahiskalioglu et al., 2017; Mohamed, 2015; Sun et al., 2017; von Bormann et al., 2014; William et al., 2001)) depending upon the circumstances. However, in neonatal and pediatric anesthesia, the use of supplemental oxygen is common in order to avoid hypoxia, as has been described in a number of reviews (Bang, 2015; Dikmen and Onur, 2017; Sola, 2008). Our results indicate that in such cases anesthesia may lead to vastly greater increase in cerebral oxygenation than would be expected from the inspired concentration of oxygen alone.
The pathogenic potential of hyperoxia is well-known (Basaga, 1990; Beal, 1995; LeBel and Bondy, 1991; Ratan et al., 1994). Damage to the brain is believed to be due to oxidative stress (Felderhoff-Mueser et al., 2004) when increased levels of reactive oxygen species (ROS) overwhelm the brain’s antioxidant capacity (Macri et al., 2010). Furthermore, it has been found that antioxidant defense in children is weaker than in adults (Friel et al., 2004; Gitto et al., 2009), which would increase their vulnerability to ROS for equivalent oxygen concentrations. The link between anesthesia and brain oxygen level has not been extensively studied, especially in neonates. Boscolo et al. (Boscolo et al., 2013) showed that isoflurane and 24% oxygen produced a 30% increase in ROS in 7-day-old rats. Yonamine et al (Yonamine et al., 2013) showed that sevoflurane and 30% oxygen induced oxidative stress in 6-day-old mice. However, without performing direct measurement of brain tissue oxygen it is difficult to rule out the possibility that the elevated ROS level could be related to hypoxia, another potential source of oxidative stress (Lu et al., 2015). Using retrospective data from cerebral/somatic oximeter measurements, Gupta et al. (Gupta et al., 2014) showed that sevoflurane anesthesia in children up to 4 years of age caused greater cerebral “hyperoxygenation” than fentanyl, which echoes a previous comparison between sevoflurane and propofol (Gupta et al., 2012). Hoffman at al. (Hoffman et al., 1997) also showed that PO2 in brain tissue increased in proportion to desflurane concentration in patients during vascular surgery. We note that oxygen regulation in the brain has been shown to be consistent among human and laboratory mammals (Dagal and Lam, 2009; Masamoto and Tanishita, 2009; Ndubuizu and LaManna, 2007). Thus, our results point to a need to examine more thoroughly the relationship between anesthesia exposure during infancy, hyperoxia, and the development of pathologies that may be connected to oxidative stress.
The high degree of individual variability indicated by standard error of the mean that was observed during 80% inspired oxygen, both in brain tissue PO2 and respiratory rate, underscores the potential risk for hyperoxia during anesthesia. The respiration in the awake state under 80% O2 also varied considerably, from a 46% to a 7% decrease, which indicates that some of the rabbits controlled oxygen intake better than others. Together, these changes indicate that anesthesia disrupts the mechanisms—both local mechanisms and possibly autoregulation as well—which normally work to maintain the oxygen level in the brain. These results also suggest that when intubation and mechanical ventilation are used, as is commonly the case during surgeries, the brain PO2 increase could be even greater, as the suppression of respiration that we observed in our experiments would not occur. Indeed, clinical reports suggest that in many cases blood PO2 can reach 120 mmHg or more without adjustment of ventilation rate (de Graaff et al., 2011). Even in the awake state, without modulation from anesthesia, the magnitude of the PO2 increase during 80% inspired oxygen indicates that brain oxygen regulation was not sufficient to maintain brain tissue oxygen in spite of decreased respiration.
The PO2 fluctuations measured by the power spectrum in the awake neonate brain were characterized by a dominance of very low frequencies (0-7 cpm), in sharp contrast to the adult brain (Linsenmeier et al., 2016) in which higher frequencies (0-20 cpm with a peak frequently near 10 cpm) appear more prevalent. Such fluctuations in brain tissue PO2 are generally attributed to spontaneous changes of vascular tone (i.e., vasomotion) (Aalkjaer et al., 2011; Hudetz et al., 1998). The most notable change occurred during anesthesia in air, when higher frequencies (25-40 cpm) appeared, which were not present during 80% inspired oxygen. Based on their frequencies, these new peaks most likely are related to respiratory-dependent changes in PO2. Surprisingly, these peaks were not especially visible in awake state, probably because they were mitigated by cerebrovascular autoregulation in response to respiration-related hemodynamic changes (Michard, 2005). Anesthesia delivery in 80% O2 mitigated these changes because of increased oxygen concentration in the brain or depressed respiration. These findings demonstrate that the PO2 power spectrum can potentially reflect underlying developmental differences in the cerebrovascular system between neonates and adults, and changes in spontaneous fluctuation provide an indicator of the functioning of oxygen autoregulation in the brain.
Neuronal activity as measured by single unit firing decreased during both sevoflurane and isoflurane, with a larger decrease during sevoflurane, as expected from the GABA-agonistic properties of isoflurane and sevoflurane (Nishikawa and Harrison, 2003). However, there are also key differences between these drugs which may explain the changes that we observed. For example, sevoflurane has lower solubility than isoflurane, and consequently a faster uptake and elimination (Stachnik, 2006). It is likely for this reason that sevoflurane produces faster recovery of neuronal activity compared to isoflurane. The deeper suppression of neuronal activity by sevoflurane, compared to isoflurane, may reflect the differences in the effects of these drugs on glutamate receptors (Vinje et al., 2002). The full spectrum of neuronal changes induced by these drugs is not entirely understood, but differential effects also have been reported in an EEG study in children (Lo et al., 2009). Overall, our data indicate that full neuronal recovery begins no earlier than 10 min after the end of anesthetic delivery for the majority of single units. Interestingly, extensive subject movements began after approximately 8-9 min and do not appear to require full neuronal recovery of all single units in this brain region. Neuronal activity across a variety of recorded cells typically decreased gradually (fig. 3), starting almost immediately after the onset of anesthesia, until reaching a steady-state plateau after approximately 4 min. Supplemental oxygen did not produce any further change in this steady state, which demonstrates that, as would be expected, single unit activity does not depend on the brain tissue PO2 as long as the brain is not in a hypoxic state.
While anesthesia depressed neuronal activity under all conditions, the extent of depression did not depend on the baseline activity in the awake state. This behavior indicates that neurons do not necessarily exhibit the same sensitivity to anesthesia. Different cells, as indicated by their different firing rates, do not experience an equivalent decrease in spiking frequency. As sevoflurane and isoflurane are both primarily GABA-agonists (Nishikawa and Harrison, 2003), this variation among cells may reflect differences in the functional diversity of GABA-receptors on neurons (Xiang et al., 1998). Furthermore, it has been found that GABA can produce mixed hyperpolarizing/depolarizing effects in the developing cortex, and anesthesia could result in a more complex pattern of neuronal changes in the brains of neonates (Kirmse et al., 2015). It is notable that whereas the mean increase in PO2 was observed under isoflurane in air but not under sevoflurane in air, sevoflurane produced a greater decrease in neuronal activity, which suggests that consumption of oxygen is smaller during sevoflurane. This difference between PO2 increase and depression of single unit activity is probably due to greater respiratory depression under sevoflurane.
Conclusion
In conclusion, our findings show that general anesthetics can increase brain tissue PO2 significantly in neonates, especially when combined with supplemental oxygen. Indeed, the PO2 increase under anesthesia delivered with 80% oxygen was vastly greater than during respiration of 80% oxygen alone. Although isoflurane and sevoflurane belong to the same class of anesthetics, notable differences were observed in their effects on neuronal activity as well as spontaneous respiration. Together these changes demonstrate the complex array of effects that anesthetics can produce upon neurons as well as on the mechanisms of oxygen regulation. While in practice clinical anesthesia typically focuses on the avoidance of hypoxia, our findings point to the need to consider the potential effects of hyperoxia as well when supplemental oxygen is utilized, particularly in children and neonates. One approach that has seen increasing adoption in clinical practice is the use of near-infrared spectroscopy (NIRS)-based cerebral oximetry (Denault et al., 2007; Murkin and Arango, 2009) to monitor non-invasively cerebral oxygenation so that FiO2 can be adjusted accordingly. Although NIRS has some limitations (for review see (Murkin and Arango, 2009), it could serve as an effective safeguard for cerebral function, especially in neonates (Martini and Corvaglia, 2018), by helping to maintain brain tissue oxygen within a safe range.
Examines how anesthesia affects brain tissue oxygen regulation in neonate rabbits.
Tissue oxygen and single units are recorded in awake and anesthetized states.
Isoflurane and Sevoflurane are tested when delivered in air vs. 80% oxygen.
Anesthesia increased brain tissue oxygen, especially with 80% inspired O2.
Each drug produced different profiles of suppression and recovery in single units.
Acknowledgments
This work was supported by National Institute of General Medical Sciences [grant number R01GM112715]
Abbreviations
- PO2
Oxygen Partial Pressure
- ROS
Reactive Oxygen Species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest
The authors declare no conflict of interest.
References
- Aalkjaer C, Boedtkjer D, Matchkov V. Vasomotion - what is currently thought? Acta Physiol (Oxf) 2011;202:253–269. doi: 10.1111/j.1748-1716.2011.02320.x. [DOI] [PubMed] [Google Scholar]
- Ahiskalioglu A, Ince I, Ahiskalioglu EO, Oral A, Aksoy M, Yigiter M, Celikkaya ME, Salman AB. Is Neuromuscular Blocker Necessary in Pediatric Patients Undergoing Laparoscopic Inguinal Hernia Repair with Percutaneous Internal Ring Suturing? Eur J Pediatr Surg. 2017;27:263–268. doi: 10.1055/s-0036-1587329. [DOI] [PubMed] [Google Scholar]
- Aksenov D, Eassa JE, Lakhoo J, Wyrwicz A, Linsenmeier RA. Effect of isoflurane on brain tissue oxygen tension and cerebral autoregulation in rabbits. Neurosci Lett. 2012;524:116–118. doi: 10.1016/j.neulet.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Aksenov DP, Li L, Miller MJ, Iordanescu G, Wyrwicz AM. Effects of anesthesia on BOLD signal and neuronal activity in the somatosensory cortex. J Cereb Blood Flow Metab. 2015;35:1819–1826. doi: 10.1038/jcbfm.2015.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang SR. Neonatal anesthesia: how we manage our most vulnerable patients. Korean J Anesthesiol. 2015;68:434–441. doi: 10.4097/kjae.2015.68.5.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaga HS. Biochemical aspects of free radicals. Biochem Cell Biol. 1990;68:989–998. doi: 10.1139/o90-146. [DOI] [PubMed] [Google Scholar]
- Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- Bosch L, Fernandez-Candil J, Leon A, Gambus PL. Influence of general anaesthesia on the brainstem. Rev Esp Anestesiol Reanim. 2017;64:157–167. doi: 10.1016/j.redar.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Boscolo A, Milanovic D, Starr JA, Sanchez V, Oklopcic A, Moy L, Ori CC, Erisir A, Jevtovic-Todorovic V. Early exposure to general anesthesia disturbs mitochondrial fission and fusion in the developing rat brain. Anesthesiology. 2013;118:1086–1097. doi: 10.1097/ALN.0b013e318289bc9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenet O, Granry JC, Poirier N, Le Gall R. -The effect of desflurane on cerebral blood flow velocity and cerebrovascular reactivity to CO2 in children. Ann Fr Anesth Reanim. 1998;17:227–233. doi: 10.1016/s0750-7658(98)80004-x. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage 23 Suppl. 2004;1:S220–S233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Crosby G, Davis PJ. General anesthesia in infancy is associated with learning disabilities-or not. Anesth Analg. 2013;117:1270–1272. doi: 10.1213/ANE.0b013e3182a9667f. [DOI] [PubMed] [Google Scholar]
- Dagal A, Lam AM. Cerebral autoregulation and anesthesia. Curr Opin Anaesthesiol. 2009;22:547–552. doi: 10.1097/ACO.0b013e32833020be. [DOI] [PubMed] [Google Scholar]
- de Graaff AE, Dongelmans DA, Binnekade JM, de Jonge E. Clinicians’ response to hyperoxia in ventilated patients in a Dutch ICU depends on the level of FiO2. Intensive Care Med. 2011;37:46–51. doi: 10.1007/s00134-010-2025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denault A, Deschamps A, Murkin JM. A proposed algorithm for the intraoperative use of cerebral near-infrared spectroscopy. Semin Cardiothorac Vasc Anesth. 2007;11:274–281. doi: 10.1177/1089253207311685. [DOI] [PubMed] [Google Scholar]
- Dikmen Y, Onur A. Perioperative hyperoxia: perhaps a malady in disguise. Rom J Anaesth Intensive Care. 2017;24:53–56. doi: 10.21454/rjaic.7518.241.yal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood GM, Tanaka A, Bellomo R. Cerebral oxygenation in mechanically ventilated early cardiac arrest survivors: The impact of hypercapnia. Resuscitation. 2016;102:11–16. doi: 10.1016/j.resuscitation.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Edmark L, Kostova-Aherdan K, Enlund M, Hedenstierna G. Optimal oxygen concentration during induction of general anesthesia. Anesthesiology. 2003;98:28–33. doi: 10.1097/00000542-200301000-00008. [DOI] [PubMed] [Google Scholar]
- Felderhoff-Mueser U, Bittigau P, Sifringer M, Jarosz B, Korobowicz E, Mahler L, Piening T, Moysich A, Grune T, Thor F, Heumann R, Buhrer C, Ikonomidou C. Oxygen causes cell death in the developing brain. Neurobiol Dis. 2004;17:273–282. doi: 10.1016/j.nbd.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, Warner DO. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–1061. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel JK, Friesen RW, Harding SV, Roberts LJ. Evidence of oxidative stress in full-term healthy infants. Pediatr Res. 2004;56:878–882. doi: 10.1203/01.PDR.0000146032.98120.43. [DOI] [PubMed] [Google Scholar]
- Gitto E, Pellegrino S, Gitto P, Barberi I, Reiter RJ. Oxidative stress of the newborn in the pre- and postnatal period and the clinical utility of melatonin. J Pineal Res. 2009;46:128–139. doi: 10.1111/j.1600-079X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ. Autonomic nervous system control of the cerebral circulation. Handb Clin Neurol. 2013;117:193–201. doi: 10.1016/B978-0-444-53491-0.00016-X. [DOI] [PubMed] [Google Scholar]
- Gupta D, Bzeih R, Zestos MM. Cerebral “hyperoxygenation” with inhalational induction of anesthesia in children: a retrospective comparison between vasoparalytic sevoflurane vs. vasoneutral fentanyl. Middle East J Anaesthesiol. 2014;22:457–466. [PubMed] [Google Scholar]
- Gupta D, Sangha J, Kaminski E. Inhalational induction with “vasoparalytic” sevoflurane: are we “hyperoxygenating” while anesthetizing developing brains? A case series discussion. Middle East J Anaesthesiol. 2012;21:863–867. [PubMed] [Google Scholar]
- Hoffman WE, Charbel FT, Edelman G. Desflurane increases brain tissue oxygenation and pH. Acta Anaesthesiol Scand. 1997;41:1162–1166. doi: 10.1111/j.1399-6576.1997.tb04859.x. [DOI] [PubMed] [Google Scholar]
- Hudetz AG, Biswal BB, Shen H, Lauer KK, Kampine JP. Spontaneous fluctuations in cerebral oxygen supply. An introduction. Adv Exp Med Biol. 1998;454:551–559. doi: 10.1007/978-1-4615-4863-8_66. [DOI] [PubMed] [Google Scholar]
- Jia F, Yue M, Chandra D, Homanics GE, Goldstein PA, Harrison NL. Isoflurane is a potent modulator of extrasynaptic GABA(A) receptors in the thalamus. J Pharmacol Exp Ther. 2008;324:1127–1135. doi: 10.1124/jpet.107.134569. [DOI] [PubMed] [Google Scholar]
- Kaiser JR, Gauss CH, Williams DK. The effects of hypercapnia on cerebral autoregulation in ventilated very low birth weight infants. Pediatr Res. 2005;58:931–935. doi: 10.1203/01.pdr.0000182180.80645.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmse K, Kummer M, Kovalchuk Y, Witte OW, Garaschuk O, Holthoff K. GABA depolarizes immature neurons and inhibits network activity in the neonatal neocortex in vivo. Nat Commun. 2015;6:7750. doi: 10.1038/ncomms8750. [DOI] [PubMed] [Google Scholar]
- LeBel CP, Bondy SC. Oxygen radicals: common mediators of neurotoxicity. Neurotoxicol Teratol. 1991;13:341–346. doi: 10.1016/0892-0362(91)90081-7. [DOI] [PubMed] [Google Scholar]
- Lee JH, Zhang J, Wei L, Yu SP. Neurodevelopmental implications of the general anesthesia in neonate and infants. Exp Neurol. 2015;272:50–60. doi: 10.1016/j.expneurol.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier RA, Aksenov DP, Faber HM, Makar P, Wyrwicz AM. Spontaneous Fluctuations of PO2 in the Rabbit Somatosensory Cortex. Adv Exp Med Biol. 2016;876:311–317. doi: 10.1007/978-1-4939-3023-4_39. [DOI] [PubMed] [Google Scholar]
- Lo SS, Sobol JB, Mallavaram N, Carson M, Chang C, Grieve PG, Emerson RG, Stark RI, Sun LS. Anesthetic-specific electroencephalographic patterns during emergence from sevoflurane and isoflurane in infants and children. Paediatr Anaesth. 2009;19:1157–1165. doi: 10.1111/j.1460-9592.2009.03128.x. [DOI] [PubMed] [Google Scholar]
- Lu Q, Harris VA, Kumar S, Mansour HM, Black SM. Autophagy in neonatal hypoxia ischemic brain is associated with oxidative stress. Redox Biol. 2015;6:516–523. doi: 10.1016/j.redox.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luginbuehl IA, Karsli C, Bissonnette B. Cerebrovascular reactivity to carbon dioxide is preserved during hypocapnia in children anesthetized with 1.0 MAC, but not with 1.5 MAC desflurane. Can J Anaesth. 2003;50:166–171. doi: 10.1007/BF03017851. [DOI] [PubMed] [Google Scholar]
- Lyons DG, Parpaleix A, Roche M, Charpak S. Mapping oxygen concentration in the awake mouse brain. Elife. 2016;5 doi: 10.7554/eLife.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri MA, D’Alessandro N, Di Giulio C, Di Iorio P, Di Luzio S, Giuliani P, Esposito E, Pokorski M. Region-specific effects on brain metabolites of hypoxia and hyperoxia overlaid on cerebral ischemia in young and old rats: a quantitative proton magnetic resonance spectroscopy study. J Biomed Sci. 2010;17:14. doi: 10.1186/1423-0127-17-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari R, Tracy M, Badawi N, Hinder M. Neonatal endotracheal intubation: How to make it more baby friendly. J Paediatr Child Health. 2016;52:480–486. doi: 10.1111/jpc.13192. [DOI] [PubMed] [Google Scholar]
- Martini S, Corvaglia L. Splanchnic NIRS monitoring in neonatal care: rationale, current applications and future perspectives. J Perinatol. 2018 doi: 10.1038/s41372-018-0075-1. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Tanishita K. Oxygen transport in brain tissue. J Biomech Eng. 2009;131:074002. doi: 10.1115/1.3184694. [DOI] [PubMed] [Google Scholar]
- Matta BF, Heath KJ, Tipping K, Summors AC. Direct Cerebral Vasodilatory Effects of Sevoflurane and Isoflurane. Anesthesiology. 1999;91:677–680. doi: 10.1097/00000542-199909000-00019. [DOI] [PubMed] [Google Scholar]
- Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology. 2005;103:419–428. doi: 10.1097/00000542-200508000-00026. quiz 449-415. [DOI] [PubMed] [Google Scholar]
- Mohamed AA. Prevention of sevoflurane agitation in children undergoing congenital hernia repair, impact of adding dexmedetomidine to caudal analgesia. Egyptian Journal of Anaesthesia. 2015;31:227–231. [Google Scholar]
- Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth 103 Suppl. 2009;1:i3–13. doi: 10.1093/bja/aep299. [DOI] [PubMed] [Google Scholar]
- Ndubuizu O, LaManna JC. Brain tissue oxygen concentration measurements. Antioxid Redox Signal. 2007;9:1207–1219. doi: 10.1089/ars.2007.1634. [DOI] [PubMed] [Google Scholar]
- Nishikawa K, Harrison NL. The actions of sevoflurane and desflurane on the gamma-aminobutyric acid receptor type A: effects of TM2 mutations in the alpha and beta subunits. Anesthesiology. 2003;99:678–684. doi: 10.1097/00000542-200309000-00024. [DOI] [PubMed] [Google Scholar]
- Olsen EA, Brambrink AM. Anesthetic neurotoxicity in the newborn and infant. Curr Opin Anaesthesiol. 2013;26:535–542. doi: 10.1097/01.aco.0000433061.59939.b7. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Semenza GL. Oxygen Sensing and Homeostasis. Physiology (Bethesda) 2015;30:340–348. doi: 10.1152/physiol.00022.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratan RR, Murphy TH, Baraban JM. Oxidative stress induces apoptosis in embryonic cortical neurons. J Neurochem. 1994;62:376–379. doi: 10.1046/j.1471-4159.1994.62010376.x. [DOI] [PubMed] [Google Scholar]
- Russo SG, Becke K. Expected difficult airway in children. Curr Opin Anaesthesiol. 2015;28:321–326. doi: 10.1097/ACO.0000000000000198. [DOI] [PubMed] [Google Scholar]
- Scheuer T, Sharkovska Y, Tarabykin V, Marggraf K, Brockmoller V, Buhrer C, Endesfelder S, Schmitz T. Neonatal Hyperoxia Perturbs Neuronal Development in the Cerebellum. Mol Neurobiol. 2017 doi: 10.1007/s12035-017-0612-5. [DOI] [PubMed] [Google Scholar]
- Sola A. Oxygen in neonatal anesthesia: friend or foe? Curr Opin Anaesthesiol. 2008;21:332–339. doi: 10.1097/ACO.0b013e3282f8ad8d. [DOI] [PubMed] [Google Scholar]
- Stachnik J. Inhaled anesthetic agents. Am J Health Syst Pharm. 2006;63:623–634. doi: 10.2146/ajhp050460. [DOI] [PubMed] [Google Scholar]
- Sun L. Early childhood general anaesthesia exposure and neurocognitive development. Br J Anaesth 105 Suppl. 2010;1:i61–i68. doi: 10.1093/bja/aeq302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Li Y, Sun Y, Wang X, Ye H, Yuan X. Dexmedetomidine Effect on Emergence Agitation and Delirium in Children Undergoing Laparoscopic Hernia Repair: a Preliminary Study. J Int Med Res. 2017;45:973–983. doi: 10.1177/0300060517699467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SP. Anesthesia in infancy linked to later disabilities: causation, association, or coincidence? Anesthesiology. 2009;111:1381–1382. doi: 10.1097/ALN.0b013e3181c0d72f. author reply 1384-1386. [DOI] [PubMed] [Google Scholar]
- Vinje ML, Moe MC, Valo ET, Berg-Johnsen J. The effect of sevoflurane on glutamate release and uptake in rat cerebrocortical presynaptic terminals. Acta Anaesthesiol Scand. 2002;46:103–108. doi: 10.1046/j.0001-5172.2001.00412.x. [DOI] [PubMed] [Google Scholar]
- von Bormann B, Suksompong S, Weiler J, Zander R. Pure oxygen ventilation during general anaesthesia does not result in increased postoperative respiratory morbidity but decreases surgical site infection. An observational clinical study. PeerJ. 2014;2:e613. doi: 10.7717/peerj.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William JM, Stoddart PA, Williams SA, Wolf AR. Post-operative recovery after inguinal herniotomy in ex-premature infants: comparison between sevoflurane and spinal anaesthesia. Br J Anaesth. 2001;86:366–371. doi: 10.1093/bja/86.3.366. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. GABAA receptor-mediated currents in interneurons and pyramidal cells of rat visual cortex. J Physiol. 1998;506(Pt 3):715–730. doi: 10.1111/j.1469-7793.1998.715bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonamine R, Satoh Y, Kodama M, Araki Y, Kazama T. Coadministration of hydrogen gas as part of the carrier gas mixture suppresses neuronal apoptosis and subsequent behavioral deficits caused by neonatal exposure to sevoflurane in mice. Anesthesiology. 2013;118:105–113. doi: 10.1097/ALN.0b013e318275146d. [DOI] [PubMed] [Google Scholar]


