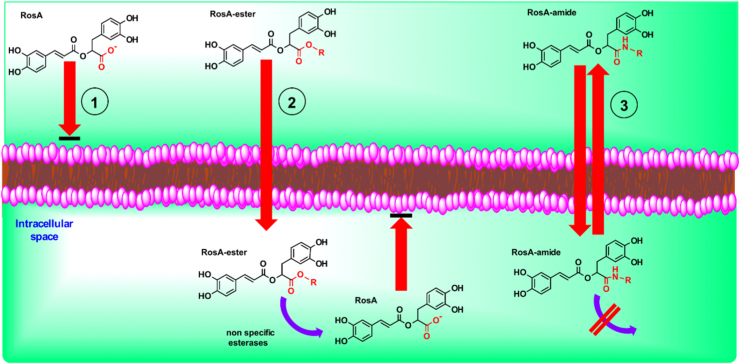
Fig. 8.
Schematic representation of diffusion and accumulation properties of RosA and its ester- and amide-derivatives. (1) Diffusion of RosA through cell membrane is hindered, due to its negative charge in the carboxilic group. (2) Lipophilic RosA-ester derivatives diffuse easily through the plasma membrane. Inside the cells, they are hydrolyzed by non-specific esterases and the generated RosA is accumulated, because it cannot diffuse out. (3) Lipophilic RosA-amide derivatives diffuse also through cell membrane, but they are not hydrolyzed (or are hydrolyzed slowly). Thus, they have sufficient time available to diffuse out again. Both parent compound and its derivatives are able to chelate intracellular labile iron in their catechol groups when present inside the cells.