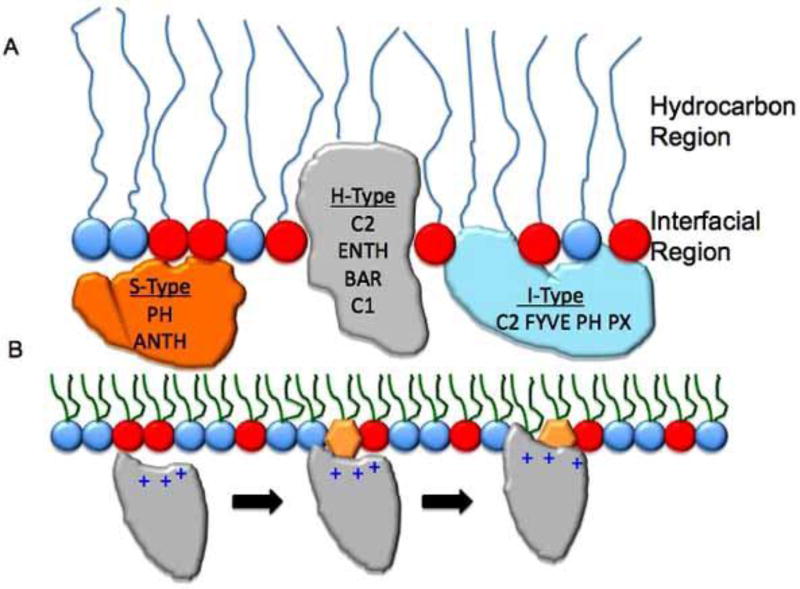
Fig. 1. Lipid binding mechanisms of lipid-binding domains.
(A) A schematic of a lipid monolayer is shown to depict the relative position of lipid-binding domains in membranes. S-type proteins shown in orange do not significantly penetrate membranes and interact primarily through electrostatics with the anionic membranes. H-type proteins shown in gray are able to penetrate into the hydrocarbon region of the bilayer due to exposed aliphatic and aromatic residues. These proteins also can interact with the interfacial region through electrostatics and H-bonding. I-type proteins partially penetrate the membrane at the interfacial phosphate region. These types of proteins generally harbor aromatic and sometimes hydrophobic residues to achieve this binding mode. Taking all three modes into consideration including the types of chemical interactions that occur at each site can serve as a building block for small molecule discovery in the field of lipid binding. (B) A general scheme of lipid binding by a peripheral protein is depicted. In this instance a PI binding domain associates weakly with anionic membranes through nonspecific electrostatic interactions. Upon binding the membrane its dimensionality is reduced as it searches for its cognate PI. The PI coordination to the binding site is responsible for inducing an electrostatic switch (reduction in the desolvation penalty) followed by insertion of aliphatic residues adjacent to the PI binding site. The combination of PI-binding and hydrophobic insertion elongates the membrane residence time of the protein and in many cases is a mode of peripheral protein activation.