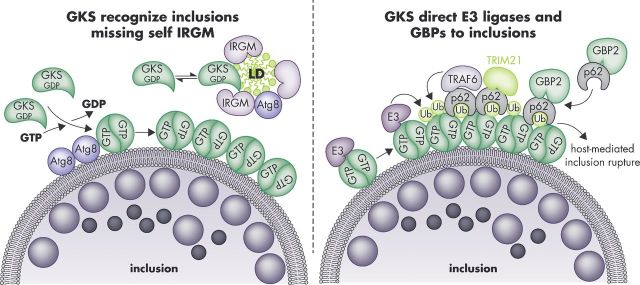
Figure 2.
Targeting of GKS proteins and GBPs to C. trachomatis inclusions in mouse cells. The GKS class of IRG proteins is guided towards inclusion membranes through a missing-self principle. The IRGM proteins Irgm1 and Irgm3 reside on ‘self’ membranes and organelles such as LDs and block GKS protein activation at these sites. The absence of these IRGM proteins from inclusions enables GTP-bound GKS dimers to form and associate with inclusion membranes. This association of GKS proteins with inclusions is further enhanced by the presence of lipidated Atg8 proteins at inclusion membranes (left panel). Inclusion-bound GKS proteins promote the recruitment of ‘pioneering’ ubiquitin E3 ligases (E3) and p62-interacting E3 ligases (TRAF6, TRIM21), which promote the decoration of inclusions with ubiquitin. Potential ubiquitination substrates are the GKS proteins themselves. The ubiquitin-binding protein p62 escorts GBP2 to inclusions (right panel). Additional p62-independent mechanisms of GBP recruitment exist (not shown). GKS-decorated inclusions rupture in a p62-dependent manner.