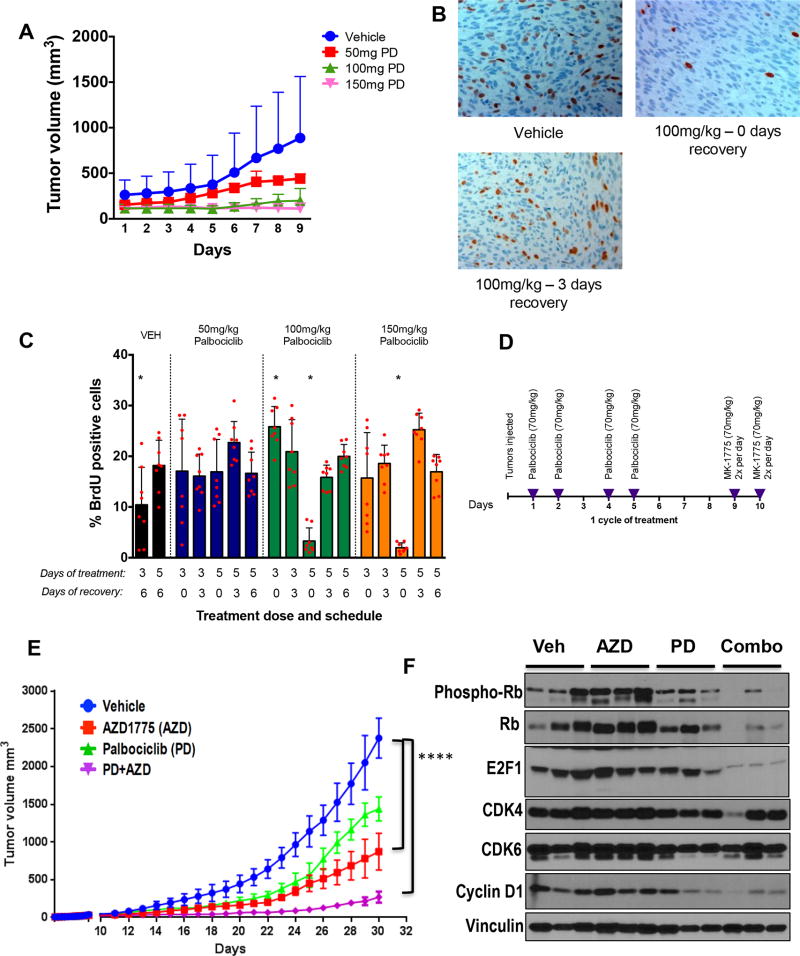
Figure 5. Optimization of in vivo treatment approach using SK-LMS1 xenografts.
(A) Average tumor volumes for mice treated with vehicle, or the indicated doses of palbociclib daily for 6 days (n=2 per arm). Tumors were measured daily with calipers. (B) Representative BrdU IHC images showing reversibility of palbociclib-induced G1 arrest. (C) Proportion of BrdU positive cells under each treatment condition. The percentage of cells that were successfully labeled with BrdU was quantified manually using at least 2 images per slide, of which 400 cells per slide were counted as either positive or negative for BrdU staining for each mouse (n=3 mice per condition) for a total of 2,400 data points for each of the bar graphs presented. (D) Schematic showing treatment schedule for combination treatment study. Palbociclib was given 4 times per week, on days 1, 2, 4 and 5 at a dose of 70 mg/kg, and AZD1775 treatment was twice per day, on days 9 and 10 to allow recovery after day 5 of palbociclib treatment. (E) Average SK-LMS1 tumor volume during treatment (n=9 vehicle arm, n=10 AZD arm, n=7 PD arm and n=7 for PD-AZD sequential combo), ****p <0.0001. (F) Western blot showing indicated proteins on tumor samples, using vinculin as a loading control. Phospho-Rb and cyclin D1 as a measure of palbociclib pharmacodynamics is significantly reduced in combination treated tumors. Levels of CDK4 and CDK6 are relatively stable across all treatment groups. Error bars represent the standard deviation from the mean, unless otherwise indicated. Pairwise comparisons were analyzed using multiple t-tests (one unpaired t-test per row).