Extended Data Figure 6.
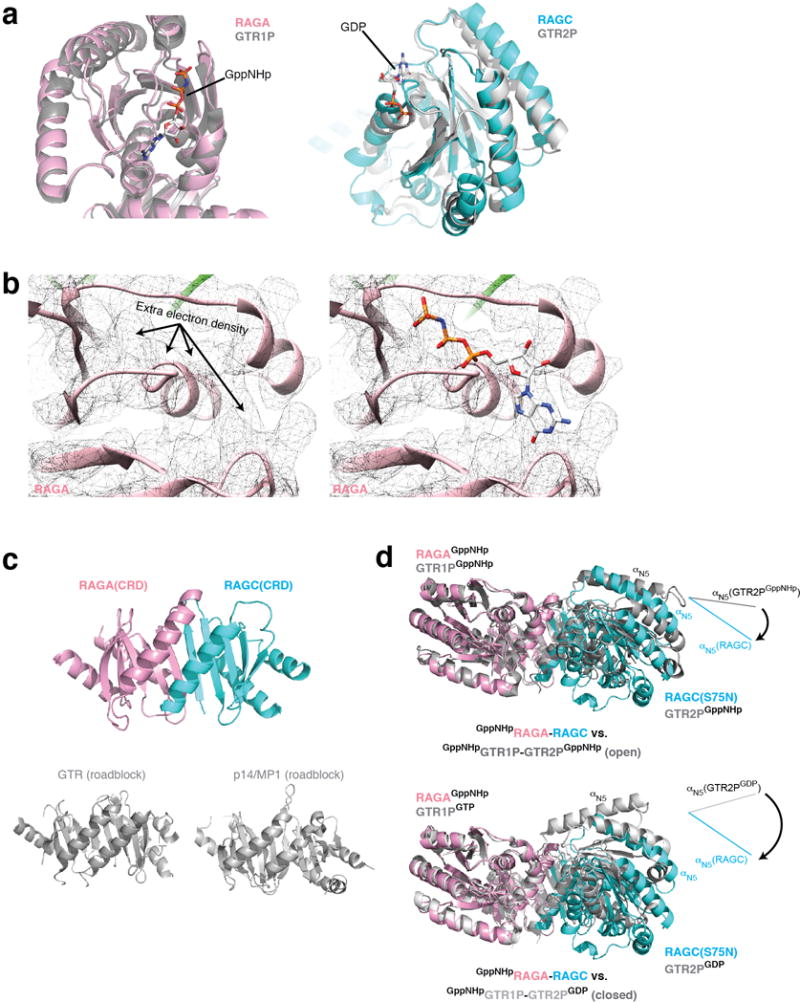
Architecture of the Rag GTPase heterodimer. a. Nucleotide binding domains (NBD) of RagA (pink) and RagC (cyan) overlap with those of Gtr1p and Gtr2p (grey). b Extra EM density can be observed in the nucleotide binding pocket of RagA, where GppNHp can be fitted. c. The C-terminal Roadblock domains (CRD) of RagA and RagC tightly dimerize with one another. For comparison, the dimerized Roadblock domains from Gtr1p-Gtr2p and p14-MP1 are shown. d. Global conformation of the Rag GTPase heterodimer in comparison to the two crystal structures of Gtr1p-Gtr2p. RagA and Gtr1p are aligned. Rotational movement of RagC-NBD is illustrated and compared with Gtr2p-NBD by the direction of αN5. The NBDs of the Rag GTPases rotate further away from one another even compared with the open conformation of Gtr1p-Gtr2p (top).
