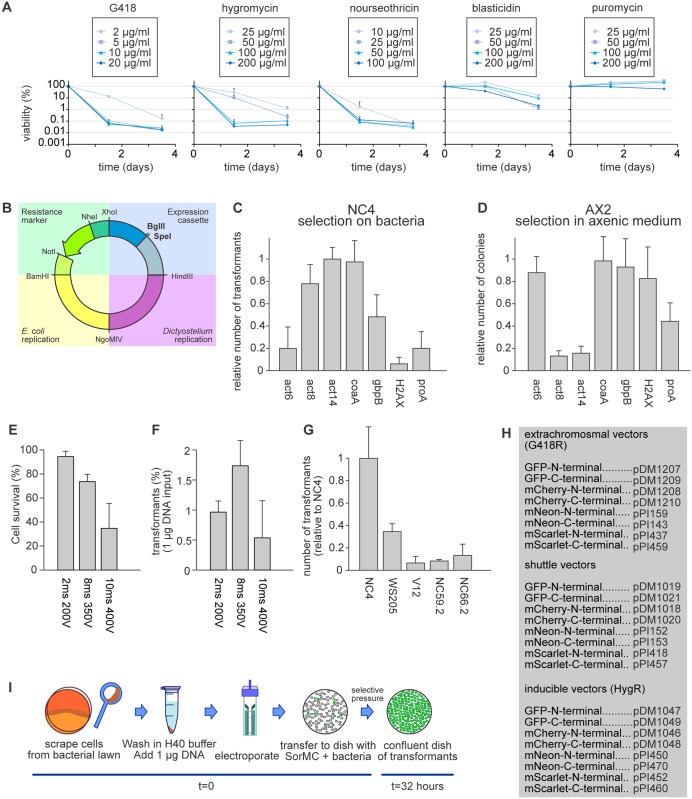
Fig 2. Selection and transfection conditions for bacterially-grown cells.
(A) Antibiotic sensitivity of bacterially-grown cells. Cells growing in petri dishes with K. pneumoniae bacteria in SorMC buffer were treated with the indicated antibiotics. Individual wells were harvested after 36 and 84 hours and the cells plated clonally on SM agar plates to determine viability (error bars are SD). (B) Schematic overview of the extrachromosomal expression vector, with the four modules shown in different colouring: green = resistance marker; blue = expression cassette; purple = Dictyostelium replication module; yellow = E. coli replication module. (C-D) The efficiencies of different promoters at driving the selectable marker in bacterially-cultured cells. Cells were transfected with 1 μg of respective plasmid and the number of colonies counted after 2 days (n = 3; error bars are SD). (E-F) Optimization of electroporation conditions. The survival of cells and number of transfectants obtained after electroporation with 2 square waves, 1 second apart, at the indicated voltages and pulse lengths. Survival was determined by clonal plating on SM agar. (H) Table of extrachromosmal plasmids in the new pDM/pPI system, including inducible and shuttle vectors. (I) Workflow of a non-axenic transfection using an extrachromosomal expression plasmid. If not indicated otherwise, NC4 cells were used in all experiments.