Fig. 3. HaloTag folding is more efficient during in vitro translation than after refolding.
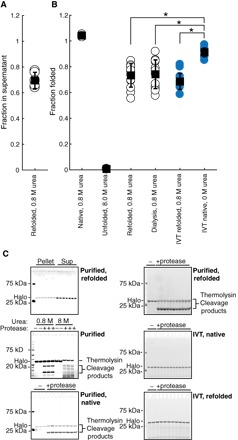
(A) Fraction of total protein remaining in supernatant after centrifugation following refolding of HaloTag to 0.8 M urea. (B) Fraction folded as measured by pulse proteolysis in conditions as indicated—either after refolding, after in vitro translation, or both. Blue circles are in vitro–translated protein. (C) Representative gels for (A) and (B). All error bars are the SDs of at least 15 separate experiments except for HaloTag in 0.8 and 8.0 M urea, which are the SDs of three experiments. *P < 0.01, Student’s unpaired t test.
