Figure 2.
RNF12 Protein Stability, Dimerization, and Nuclear Localization Are Unaltered by XLID-Associated Mutations
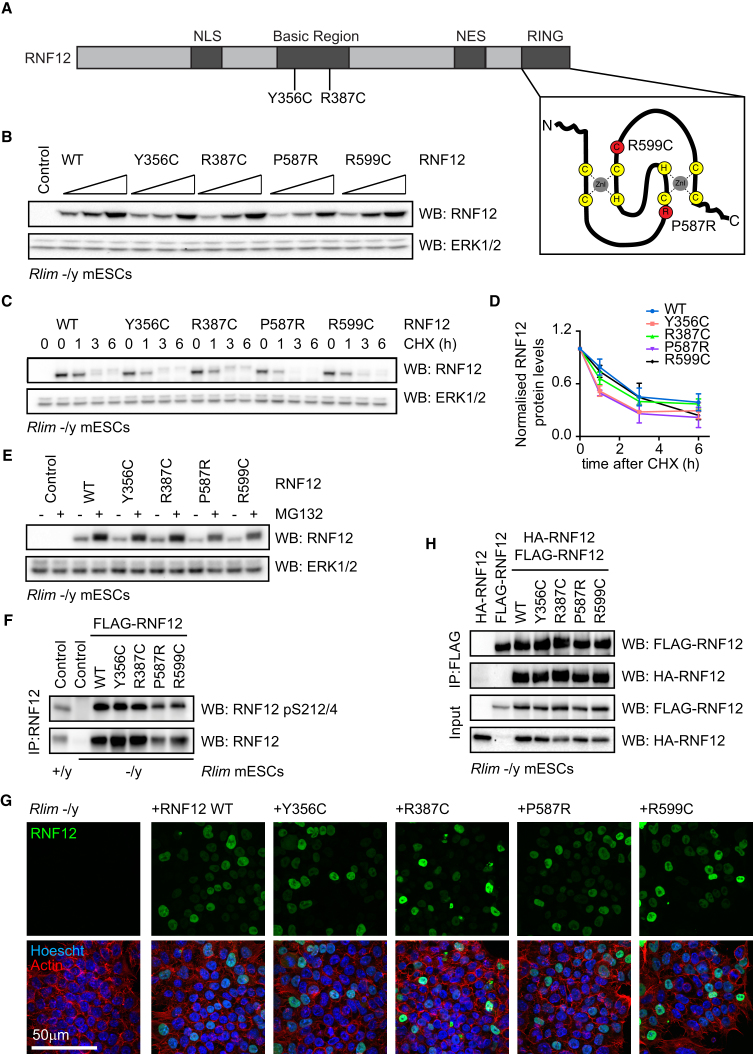
(A) Schematic of RNF12 indicating location of XLID mutations. NLS, nuclear localization signal; NES, nuclear export signal. Inset: positions of XLID mutations (red circles) within the RNF12 RING domain. Adapted from Metzger et al. (2014).
(B) Rlim −/y ESCs were transfected with WT human RNF12 or XLID mutants, and RNF12 and ERK1/2 levels were determined by immunoblotting.
(C) Rlim −/y ESCs were transfected with WT RNF12 or XLID mutants and treated with 350 μM cycloheximide (CHX) for the indicated times. RNF12 and ERK1/2 levels were determined by immunoblotting.
(D) Quantification of western blot signals shown in (C). Data are represented as mean ± SEM (n = 3).
(E) Rlim −/y ESCs were transfected with indicated vectors and treated with 10 μM MG132 for 6 hr. RNF12 and ERK1/2 levels were determined by immunoblotting.
(F) Rlim +/y or Rlim −/y ESCs were transfected with the indicated constructs, and RNF12 NLS phosphorylation at Ser212/Ser214 and total RNF12 were analyzed by immunoblotting.
(G) ESCs treated as in (F) were fixed, and subcellular localization of WT RNF12 or XLID mutants was analyzed via immunofluorescence and confocal microscopy.
(H) Rlim −/y ESCs were transfected with N-terminal FLAG- or HA-tagged WT RNF12 or XLID mutants and lysates subjected to FLAG co-immunoprecipitation and immunoblot analysis with FLAG or HA antibodies.
See also Figure S2.