Abstract
Milkfish (Chanos chanos), which is resistant to water quality changes is the fourth largest aquaculture commodity. Abandoned wastes of fish scale and bones aggravate environmental pollution. In this study, the effect of collagen peptides isolated from milkfish scales (MSCP) by pepsin-soluble collagen method on cell viability was investigated. The antioxidant, anti-inflammatory, and DNA-protective activities of MSCP were also evaluated. Results revealed that more than 95% of viable cells were retained in human keratinocytes after addition of 100 mg/mL MSCP. Measurement of DPPH· and ABTS· + radical scavenging activities and cellular reactive oxygen species revealed the high antioxidant activities of MSCP. MSCP demonstrated anti-inflammatory activities by reducing lipoxygenase activity and nitric oxide (NO·) radicals. Moreover, DNA electrophoresis assay indicated that MSCP treatment can directly protect against cyclobutane di-pyrimidine production and DNA single-strand breaks, which are harmful effects of UV radiation and H2O2. Given its antioxidant, anti-inflammatory, and DNA-protective activities, MSCP has potential applications in cosmeceuticals and supplementary health food.
Keywords: Milkfish, Fish scale, Collagen peptide, Antioxidant, Anti-inflammation
Introduction
Collagen, a protein that consists of three polypeptide chains with a triple-helix molecular structure and three repeated residues of (Gly-X–Y)n, can be found in connective tissues such as skin, bones, tendons, and cartilages (Ramshaw et al. 1998). Several crystal structures of collagen have been analyzed and deposited in the RCSB protein data bank. This protein displays several bioactive properties, including good biocompatibility, biodegradability, non-immunogenicity, and low antigenicity; it also promotes cell proliferation and attachment (Venkatesan et al. 2017). Collagen peptides hydrolyzed into di-and tripeptides by collagenase from nonpathogenic Bacillus species are resistant to further digestion by proteases or peptidases; the di-and tripeptides (Pro-Hyp and Gly-Pro-Hyp) are stable in gastrointestinal fluid and rat plasma for short-term retention (Sontakke et al. 2016). Additionally, collagen peptides are key ingredients in nutricosmetic products and supplementary health food because they can increase skin hydration and improve facial skin conditions (Inoue et al. 2016). In pharmaceutical applications, a hydroxyproline-containing marine collagen peptide from Alaska pollack inhibits human immunodeficiency virus (HIV-1) infection (Jang and Park 2016).
Marine fish is an important source of food for humans. However, large amounts of abandoned wastes of fish scales, skin, and bones result in the problem of environmental pollution. Therefore, high-value by-products generated from marine fish have attracted increasing attention in recent years. Collagen is the most abundant protein that can be obtained from fish scales, skin, and bones and further utilized in various applications, including cosmeceuticals, tissue engineering, and anti-diabetic medications (Silva et al. 2014; Venkatesan et al. 2017). Marine-derived collagen, an ingredient in skin care products, can scavenge free radicals. To isolate collagen from different parts of fish species, acid-soluble collagen and pepsin-soluble collagen methods are usually employed to extract and harvest collagen (Silva et al. 2014). Pepsin-soluble collagen method reportedly has higher yield than acid-soluble collagen method (Shanmugam et al. 2012; Venkatesan et al. 2017). Several studies have isolated collagen from the skin, bones, and scales of Sepiella inermis, Otolithes ruber, Magalaspis cordyla, Trachurus japonicas, and Parupeneus heptacanthus (Kumar and Nazeer 2012; Matmaroh et al. 2011; Minh Thuy le et al. 2014; Shanmugam et al. 2012). In addition, the effect of marine-derived collagen peptides on skin has been investigated. Jellyfish collagen hydrolysates protect the skin against UV radiation damage (Fan et al. 2013). Similar results were also obtained using 6 kDa collagen peptides from cod skin (Hou et al. 2012). These results clearly indicate that marine-derived collagen peptides are effective and safe to use for developing skin-targeting cosmeceuticals.
Milkfish (Chanos chanos) is a tropical marine fish that can adapt to water quality changes. It is the fourth largest aquaculture commodity in Taiwan, India, the Philippines, and Indonesia (Palanikumar et al. 2013). Over 330,000 tons of milkfish are produced annually, and the demand for milkfish has increased. However, collagen peptides from milkfish scales (MSCP) have not yet been characterized. Hence, in the present study, MSCP was extracted by pepsin-soluble collagen method. Results demonstrated the antioxidant, anti-inflammatory, and DNA-protective properties of MSCP.
Materials and methods
Collagen peptide extraction and purification
Milkfish was purchased from the mariculture of a private vendor in Tainan, Taiwan between 2013 and 2014. Fish scales were removed manually and stored at − 20 °C until used. Collagen peptides were extracted and purified using the method described by Shanmugam et al. (2012). Fish scales (10 g) were homogenized and extracted with 100 mL of 0.1 N NaOH to remove non-collagenous proteins. The insoluble residue was washed by distilled water and suspended in 50 mL of 0.5 M acetic acid with 1% (w/v) pepsin at 4 °C for 2 days. The debris was removed by centrifugation at 20,000×g for 1 h. The supernatant was then dialyzed against 2 L of 0.02 M Na2HPO4 (pH 7.2), and a new dialysate was replenished every 12 h for 3 days at 4 °C. The extract of the milkfish scales with supernatant was salted out by adding NaCl to a final concentration of 0.8 M and precipitated with 2.3 M NaCl to obtain collagen. The precipitate with collagen was obtained by centrifugation at 20,000×g for 1 h, dissolved in 50 mL of 0.5 M acetate, and dialyzed against 2 L of 0.1 M acetate for 1 day, followed by 2 L of distilled water for 1 day. The amicon ultra centrifugal filter with 3 K device (Merck Millipore, MA, USA) was utilized to collect collagen peptides of less than 3000 Da. Freeze drying was conducted to concentrate collagen peptides. All procedures were performed at 4 °C. Coomassie Brilliant Blue G-250 was used to detect the collagen peptide, and absorbance was recorded on a spectrophotometer at OD595. The collagen peptide obtained and purified from milkfish was designated as MSCP.
Total nitrogen content of MSCP
Total nitrogen was determined through macro-Kjeldahl method (Oftedal et al. 2014). Crude protein was evaluated as follows: total nitrogen × 6.38.
Cell viability of MSCP by MTT assay
Cell viability was assessed by 3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay using the method described by Lopez-Garcia et al. (2014). HaCaT cells (1 × 105/mL) were inoculated in a 96-well plate and incubated at 37 °C under 5% CO2 for 24 h. Various concentrations of MSCP between 0 and 100 mg/mL were added to HaCaT cells for 24 h, and fresh medium with MTT was displaced at 37 °C with 5% CO2 for 4 h incubation. Absorbance was recorded on an enzyme-linked immunosorbent assay (ELISA) reader at OD570 (BioTek, Synergy™2, USA).
Antioxidant activity of MSCP by DPPH· radical scavenging assay
Antioxidant activity was evaluated with 2,2-diphenyl-1-picrylhydrazyl (DPPH·) radical scavenging assay by using the method described by Bersuder et al. (1998). In brief, 4 mL of 0.5, 1, and 10 mg/mL MSCP was mixed with 1 mL of 10 mM DPPH· (dissolved in methanol to a final concentration of 2 mM). The mixture was left in the dark at room temperature for 30 min. The remaining DPPH· radical was determined on a spectrophotometer at OD517. Distilled water (4 mL) was used to substitute for MSCP sample as background (absorption control). Ascorbic acid was adopted as positive control. DPPH· radical scavenging activity was calculated as follows:
where the control was treated with distilled water.
Antioxidant activity of MSCP by ABTS· + radical scavenging assay
Antioxidant activity was determined through ABTS· + radical scavenging assay by using the method described by Re et al. (1999). ABTS· + radical solution was prepared by oxidation of reagents with peroxidase (44 U/mL), 75 μM H2O2, 750 μM ABTS· + , and H2O with a volume ratio of 1:1:1:6. The mixture was placed in the dark at room temperature for 1 h. For antioxidant activity determination, 180 μL of the ABTS· + radical solution was added with 20 μL of samples of different-concentrations (0.5, 1, and 10 mg/mL) and incubated for 10 min. Absorbance was recorded on a spectrophotometer at OD620. Trolox (10 mg/mL) was used as positive control. ABTS· + radical scavenging activity was calculated as follows:
Reactive oxygen species (ROS) production
HaCaT cells (1 × 105/mL) were inoculated in a 96-well plate and incubated for 24 h. The cells were added with 0.5 and 1 mg/mL MSCP and allowed to react for 24 h. The cells were washed with PBS, and added with 0.1 mM H2O2 to induce ROS generation for 1 h. The supernatant was removed, and the cells were washed with PBS. The cells were then placed in 100 μL of fresh medium with 1 μL of DCFH2-DA (10 μM) and incubated at 37 °C for 30 min in the dark. Afterward, the supernatant was discarded, and the filtrate was added with 100 μL of PBS for ELISA (BioTek, Synergy™2, USA) with excitation at 504 nm and emission at 524 nm. Fluorescence intensity was calculated.
Anti-inflammatory activity of MSCP by lipoxygenase
The anti-inflammatory activity using lipoxygenase was measured as described Khalili et al. (2009). In brief, 15 mM linoleic acid was prepared in 10 mL of Tris–HCl buffer (0.1 M, pH 9) containing 50 mg of Tween by ultrasonic dispersion. The mixture was added with 250 µL of 1 M NaOH and Tris–HCl buffer to obtain a final concentration of 10 mM linoleic acid solution. Lipoxygenase activity was evaluated using the mixture of Tris–HCl buffer (0.1 M, pH 9.0, 95.5 µL), MSCP (0.5 and 1 mg/mL, 1 µL), lipoxygenase isoenzyme (Lox-1, 135 U, 2 µL), and linoleic acid solution as substrate (10 mM, 1.5 µL). Lipoxygenase catalyzes the conversion of linoleic acid into conjugated hydroperoxide, which can be detected at 234 nm. Thus, inhibition of Lox-1 activity was determined by spectrophotometry at OD234. Caffeic acid and buffer served as positive and negative control, respectively. Inhibition of lipoxygenase was calculated as follows:
Nitric oxide (NO·) radical production
NO· was detected using the method described by Ravishankara et al. (2002) with minor modifications. MSCP (2 µL) of different concentrations (0.5 and 1 mg/mL) was mixed with 98 µL of sodium nitroprusside (5 mM) at 25 °C for 150 min. The sample was then added with 100 µL of Griess Reagent (0.1% naphthylenediamine dihydrochloride, 5% phosphoric acid, and 1% sulfanilamine) for spectrophotometric determination at OD560.
DNA protection assay
The protective effect of MSCP on DNA was investigated using pUC119 plasmid as supercoil form. The plasmid and MSCP (500 μg/mL) were mixed and treated with 20 mJ/cm2 UVB, 1 mM H2O2, and 0.5 mM FeSO4 at 37 °C for 1 h. The mixture was subjected to electrophoresis at 100 V for 25 min on a 0.8% agarose gel.
Statistical analysis
Each analysis was conducted in triplicate independent experiments. Statistical analysis was performed using Microsoft Excel, and the results were presented with standard deviation.
Results
Collagen peptide extraction and cell viability detection
Crude protein of collagen peptide was extracted by pepsin-soluble collagen method and estimated by the Kjeldahl method. The total protein content determined by nitrogen conversion was 92.8 g protein/100 g extracted collagen peptide. In addition, no fat was detected in accordance with Folch method. This finding indicated that pepsin-soluble collagen method could obtain high amounts of collagen protein.
Cell viability was assessed using MTT test. Different doses of MSCP were utilized to detect HaCaT cell viability. Almost 100% of viable cells as MSCP lowering 50 mg/mL can be observed. The percentage of viable cells remained more than 95% even after adding 100 mg/mL MSCP. This result implies that MSCP exerts slight cytotoxicity to human keratinocytes.
Antioxidant activity of MSCP
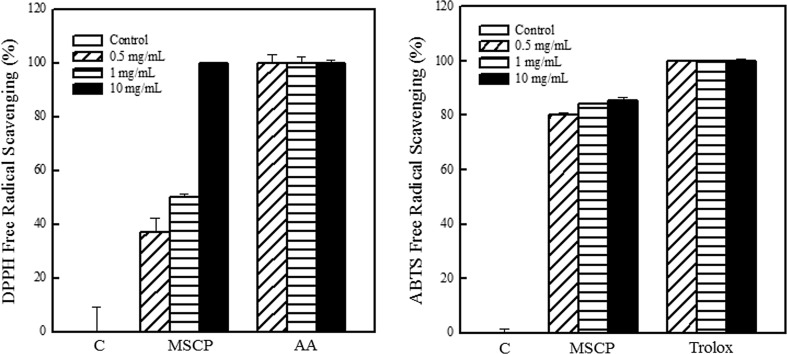
The antioxidant activity of MSCP was evaluated using DPPH· and ABTS· + radical scavenging assays and ROS reduction. Ascorbic acid and trolox were used as positive controls in the DPPH· and ABTS· + radical scavenging assays, respectively. Results showed that MSCP exhibited significant antioxidant activity. MSCP scavenged DPPH· and ABTS· + in a dose-dependent manner. MSCP (1 mg/mL) exhibited 50% inhibition rate of DPPH· radicals (Fig. 1). When the MSCP concentration was increased to 10 mg/mL, DPPH· radicals were completely arrested. The addition of MSCP also significantly inhibited ABTS· + . Over 80% scavenging rate of ABTS· + was achieved after treatment with 0.5 mg/mL MSCP.
Fig. 1.
Antioxidant activity of MSCP. Radical scavenging capability was determined by the DPPH· and ABTS· + method. Ascorbic acid (AA) and trolox were used as positive control. Results are mean ± SD (n = 3)
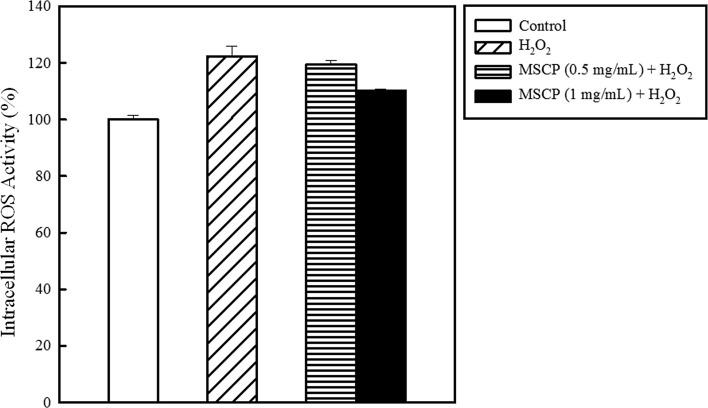
ROS are hazardous on cellular function, inducing modifications of nucleic acids, proteins, and lipids, and eventually lead to the development of aging-related diseases (Imlay 2013; Saluja and Fabi 2017). In the present study, H2O2 was applied to HaCaT cells to analyze the effect of MSCP on ROS generation. The intracellular ROS activity was increased by 22% when 0.1 mM H2O2 was added (Fig. 2). MSCP prevented ROS formation in HaCaT cells in a dose-dependent manner. Treatment with 1 mg/mL MSCP decreased the ROS activity by 12%.
Fig. 2.
Inhibition of intracellular ROS level by MSCP. Changes in the intracellular ROS level were indicated by the DCFH-DA fluorescence intensity in HaCaT cells treated with MSCP. The intracellular ROS generation level of HaCaT cells was measured by DCFH-DA, which is MSCP, to detect the produced H2O2 (0.1 mM). The control indicates the HaCaT cells without MSCP and H2O2 treatment. Each value is presented as mean ± SD from triplicate independent experiments
Anti-inflammatory activity of MSCP
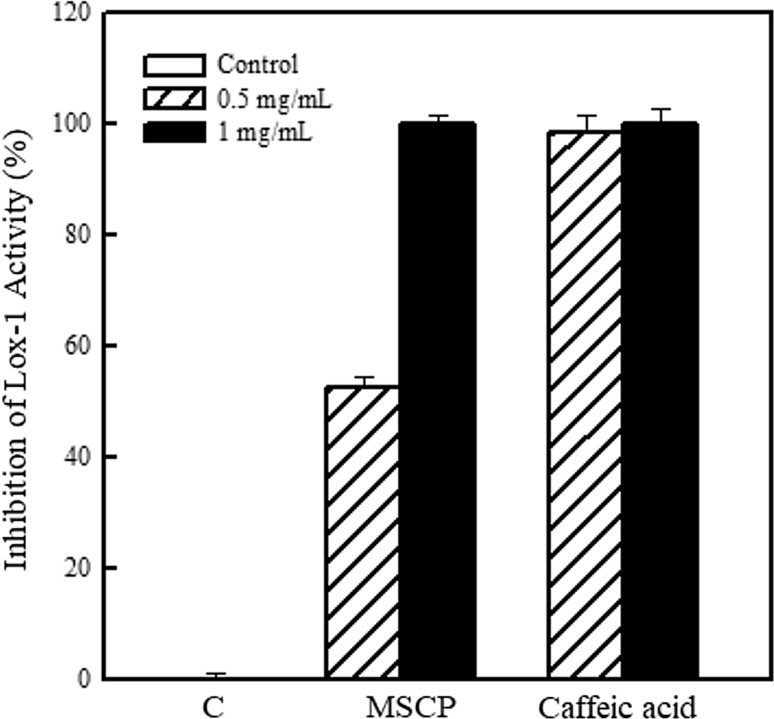
Lipoxygenase is involved in the synthesis of leukotrienes, which are inflammatory mediators of arachidonic acid (Du et al. 2017). In this regard, inhibition of lipoxygenase activity by MSCP was investigated. Caffeic acid served as positive control in the assay. Treatment with 0.5 and 1 mg/mL MSCP repressed lipoxygenase activity by 52.3 and 100%, respectively (Fig. 3). The half maximal inhibitory concentration (IC50) of MSCP for inhibiting lipoxygenase activity was calculated to be 475.9 μg/mL.
Fig. 3.
Anti-inflammatory activity of MSCP. Inhibition of lipoxygenase isoenzyme (Lox-1) with the addition of MSCP and linoleic acid was determined by a spectrophotometer at OD234. Caffeic acid was used as positive control. Results are mean ± SD (n = 3)
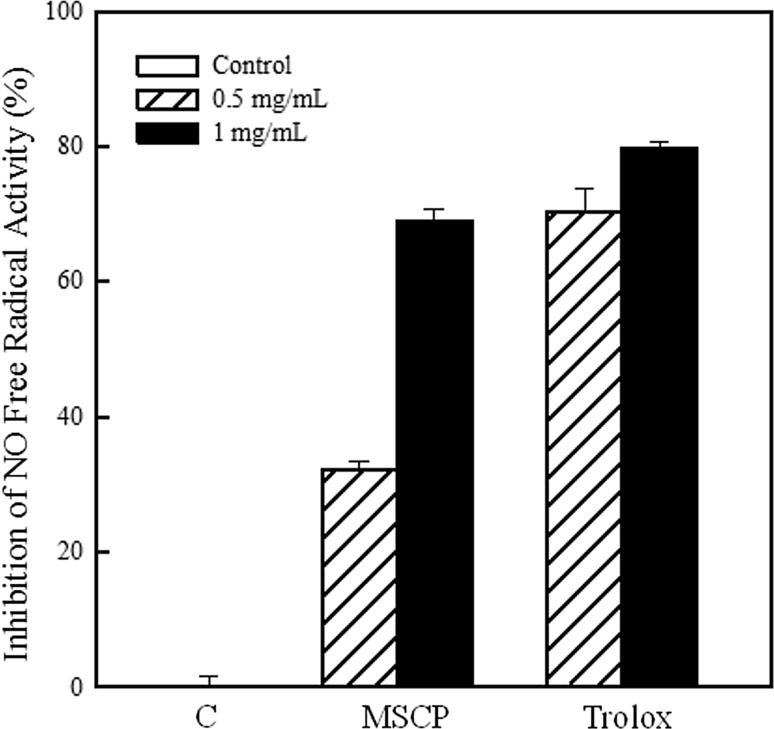
NO· is recognized as the factor involved in development of inflammation, cancer and pathological conditions (Moncada et al. 1991). NO· scavenging activities of MSCP and trolox as the positive control were compared (Fig. 4). The amounts of NO· generated were decreased by 32.2 and 68.9% after adding 0.5 and 1 mg/mL MSCP, respectively. Also, the IC50 of MSCP for repressing NO· production was calculated to be 742.5 μg/mL.
Fig. 4.
Anti-inflammatory activity of MSCP. Scavenging capability of NO· was determined. Trolox was used as positive control. Results are mean ± SD (n = 3)
DNA protection of MSCP
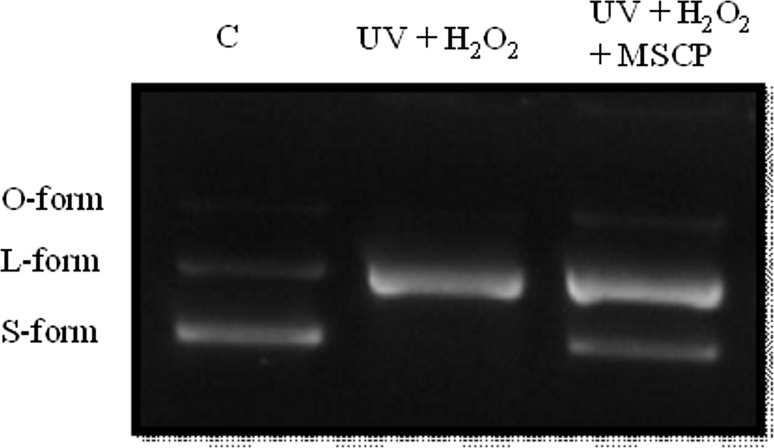
UV causes the production of cyclobutane di-pyrimidine of DNA, and H2O2 induces the generation of DNA single-strand breaks (Ray and Husain 2002). In the present study, DNA damage was simulated via UV and H2O2 treatments to evaluate the DNA-protective capability of MSCP. The pUC119 plasmid exhibited supercoil O-, L-, and S-forms without UV and H2O2 treatments as control. Meanwhile, only single-band DNA was observed in the presence of UV and H2O2 (Fig. 5). However, pUC119 plasmid with MSCP showed similar results to the control. These results indicate that MSCP significantly confers DNA protection.
Fig. 5.
Capability of MSCP to protect against DNA damage by UV and H2O2. pUC119 plasmid was treated with MSCP, 20 mJ/cm2 UVB, 1 mM H2O2, and 0.5 mM FeSO4. These DNAs were prepared by electrophoresis in 0.8% agarose
Discussion
Much attention has been focused on the use of marine fish-derived collagen in tissue engineering and wound healing (Silva et al. 2014). In addition, collagen peptides are bioactive ingredients that serve as safe functional food for improvement of skin physiology (Venkatesan et al. 2017). In the present research, collagen peptide designated as MSCP was extracted from milkfish scales. Ash and protein were the main components of fish scales. According to our result of pepsin-soluble collagen method, the productivity of MSCP was 8.3% (w/w). This result is more desirable than that reported for deep-sea redfish (Sebastes mentella) with 6.8% yield (Wang et al. 2008). Moreover, MSCP exhibited significant anti-oxidant, anti-inflammatory, and DNA-protective activities as well as safety. MSCP can scavenge DPPH· and ABTS· + radicals and reduce ROS generation. To the best of our knowledge, little research has been directed toward the anti-inflammatory and DNA-protective effects of MSCP. In the present study, MSCP exhibited a dose-dependent anti-inflammatory effect and contributed to DNA protection against UV and H2O2 damage. Hence, MSCP can serve as additive in cosmeceuticals, tissue engineering, and health food.
Collagen is generally recognized as safe and has different applications. In this study, 0–50 mg/mL MSCP exhibited no cytotoxicity. However, 100 mg/mL MSCP showed slightly cytotoxic effect, which could be due to changes in the osmolarity of the culture solution (Fan et al. 2016; Zhu et al. 2014). The cytotoxic effect of MSCP is significantly lower than those of commercial products (Zhu et al. 2014). Different polymers, including carboxymethyl cellulose, carboxymethyl chitosan, and hydroxypropyl chitosan, have been conjugated with collagen peptides for applications in pharmaceutical and food industries (Fan et al. 2014, 2016; Zhu et al. 2014). Some modifications of polymers grafted with collagen peptides, including carboxymethyl cellulose, carboxymethyl carrageenan, and hydroxypropyl chitosan, can even promote cell proliferation and cell viability. These findings reveal the definite safety benefits of collagen peptides.
Antioxidant activity is a critical factor for protection against oxidative stress. Oxidative stress is derived from ROS containing singlet oxygen species, alkyl radicals, hydroxyl radicals, peroxide radicals, and superoxide radicals. These ROS can damage cellular DNA, proteins, and lipids, thus leading to inflammation, cancer, and neuron-related diseases (Ray and Husain 2002). Antioxidant peptides from various marine fish can inhibit oxidative damage by arresting free-radical chain reactions (Fan et al. 2014). Moreover, several works have been published regarding the functions of antioxidant peptides with Cys, Tyr, and His in chelating oxidation-related ions or eliminating free radicals (Wu et al. 2015). Differently, collagen peptides, which are common antioxidant peptides, exert antioxidant ability under high amounts of Gly and Pro without forming a strong active site against free radicals (Wu et al. 2017). Thus, several studies suggested that the amino acid composition and hydrolysis degree of collagen may contribute to its antioxidant activity. In addition, antioxidant capacity of collagen peptides depends on species resources, and no unique factor that affects the antioxidant capacity of hydrolysates has been determined (Blanco et al. 2017). The results of DPPH· and ABTS· + radical scavenging assays provide evidence that MSCP possesses antioxidant capabilities (Fig. 1). MSCP was more active on ABTS· + than on DPPH· radicals. ROS activity determination also revealed that MSCP prevented H2O2 damage, which induced ROS generation (Fig. 2). MSCP could be grafted with different polymers, including carboxymethyl chitosan, hydroxypropyl chitosan, and carboxymethyl cellulose, to improve its antioxidant activity (Fan et al. 2014, 2016; Zhu et al. 2014).
Inflammation is an immune response to foreign stimuli, resulting in the restoration of normal functions. ROS may directly drive inflammation or indirectly mediate through cytoplasmic proteins that modulate inflammatory activity (Martinon et al. 2009). Previous studies reported that antioxidant peptides possess anti-inflammatory property. Lipoxygenase plays an important role in synthesis of leukotrienes, which are mediators of several allergic and inflammatory conditions (Du et al. 2017). NO· is a mediator and a regulator of inflammatory responses. Hence, the reduction of lipoxygenase activity and NO· can be applied to evaluate inflammation extent. In the present study, MSCP demonstrated a dose-dependent anti-inflammatory capability (Figs. 3, 4). The results are consistent with those of bioactive peptides from marine resources (Kim et al. 2013). The reduction and inhibition of nitric oxide and lipoxygenase, respectively, may be due to the ability of the –OH and –NH2 groups of collagen peptides to bind to nitric oxide radical and lipoxygenase (Zhu et al. 2014). Furthermore, the present work is the first to report the anti-inflammatory capability of MSCP. Consequently, MSCP can be clinically applied in patients with knee osteoarthritis, which is the most common type of arthritis in elderly populations worldwide (Kumar et al. 2015). MSCP can also be used to treat inflammation-related disorders, such as atopic dermatitis in adults, with 1–3% prevalence (Hoy 2017).
The generation of ROS and the depletion of endogenous antioxidants are induced by UV exposure, which result in the photochemical modification of genetic DNA and oxidative stress of H2O2 (Ray and Husain 2002). These risk factors enhance the development of premalignant skin lesions and skin cancer. Skin protection against UV can be divided into endogenous protection of melanin and enzymatic antioxidants, and supplementary consumption of food containing vitamins A, C, and E (Godic et al. 2014). In the present experiments, 0.5 mg/mL MSCP can directly confer DNA protection after UV and H2O2 exposure (Fig. 5). This result is consistent with the fact that MSCP exerts radical-scavenging ability and thus enables protection against DNA damage.
In conclusion, milkfish is regarded as a high value marine food, and its rate of production has been increasing yearly. A large amount of fish scales can be converted into useful collagen peptides to minimize pollution and increase the value of by-products. Our findings demonstrate that MSCP exhibits significant antioxidant, anti-inflammatory, and DNA-protective activities. Thus, collagen peptides exhibit potential as additives in health foods because of their lack of cytotoxicity.
Acknowledgements
The authors would like to thank the Xiamen Municipal Bureau of Science and Technology, Fujian, China (3502Z20153032) and the Ministry of Science and Technology, Taiwan (MOST105-2622-E-041-002-CC3 and MOST104-2314-B-037 -049-MY2).
Abbreviations
- MSCP
Milkfish scale collagen peptide
- MTT
[3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide]
- DPPH·
2,2-diphenyl-1-picrylhydrazyl
- Lox-1
Lipoxygenase isoenzyme
- IC50
Half maximal inhibitory concentration
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Yu-Pei Chen and Chia-Hua Liang have contributed equally to this work.
Contributor Information
Guey-Horng Wang, Phone: +86-592-5923631, Email: wgh@xmmc.edu.cn.
Leong-Perng Chan, Phone: +86-592-5923631, Email: oleon24@yahoo.com.tw.
References
- Bersuder P, Hole M, Smith G. Antioxidants from a heated histidine-glucose model system. I: investigation of the antioxidant role of histidine and isolation of antioxidants by high-performance liquid chromatography. J Am Oil Chem Soc. 1998;75:181–187. doi: 10.1007/s11746-998-0030-y. [DOI] [Google Scholar]
- Blanco M, Vazquez JA, Perez-Martin RI, Sotelo CG. Hydrolysates of fish skin collagen: an opportunity for valorizing fish industry byproducts. Mar Drugs. 2017;15:131. doi: 10.3390/md15050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du B, Yang Y, Bian Z, Xu B. Characterization and anti-Inflammatory potential of an exopolysaccharide from submerged mycelial culture of Schizophyllum commune. Front Pharmacol. 2017;8:252. doi: 10.3389/fphar.2017.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Zhuang Y, Li B. Effects of collagen and collagen hydrolysate from jellyfish umbrella on histological and immunity changes of mice photoaging. Nutrients. 2013;5:223–233. doi: 10.3390/nu5010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Peng M, Zhou X, Wu H, Hu J, Xie W, Liu S. Modification of carboxymethyl cellulose grafted with collagen peptide and its antioxidant activity. Carbohydr Polym. 2014;112:32–38. doi: 10.1016/j.carbpol.2014.05.056. [DOI] [PubMed] [Google Scholar]
- Fan L, Zou S, Ge H, Xiao Y, Wen H, Feng H, Liu M, Nie M. Preparation and characterization of hydroxypropyl chitosan modified with collagen peptide. Int J Biol Macromol. 2016;93:636–643. doi: 10.1016/j.ijbiomac.2016.07.093. [DOI] [PubMed] [Google Scholar]
- Godic A, Poljsak B, Adamic M, Dahmane R. The role of antioxidants in skin cancer prevention and treatment. Oxid Med Cell Longev. 2014;2014:860479. doi: 10.1155/2014/860479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou H, Li B, Zhang Z, Xue C, Yu G, Wang J, Bao Y, Bu L, Sun J, Peng Z, Su S. Moisture absorption and retention properties, and activity in alleviating skin photodamage of collagen polypeptide from marine fish skin. Food Chem. 2012;135:1432–1439. doi: 10.1016/j.foodchem.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Hoy SM. Crisaborole ointment 2%: a review in mild to moderate atopic dermatitis. Am J Clin Dermatol. 2017 doi: 10.1007/s40257-017-0327-4. [DOI] [PubMed] [Google Scholar]
- Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol. 2013;11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue N, Sugihara F, Wang X. Ingestion of bioactive collagen hydrolysates enhance facial skin moisture and elasticity and reduce facial ageing signs in a randomised double-blind placebo-controlled clinical study. J Sci Food Agric. 2016;96:4077–4081. doi: 10.1002/jsfa.7606. [DOI] [PubMed] [Google Scholar]
- Jang IS, Park SJ. Hydroxyproline-containing collagen peptide derived from the skin of the Alaska pollack inhibits HIV-1 infection. Mol Med Rep. 2016;14:5489–5494. doi: 10.3892/mmr.2016.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili M, Hasanloo T, Kazemi Tabar SK, Rahnama H. Influence of exogenous salicylic acid on flavonolignans and lipoxygenase activity in the hairy root cultures of Silybum marianum. Cell Biol Int. 2009;33:988–994. doi: 10.1016/j.cellbi.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Kim EK, Oh HJ, Kim YS, Hwang JW, Ahn CB, Lee JS, Jeon YJ, Moon SH, Sung SH, Jeon BT, Park PJ. Purification of a novel peptide derived from Mytilus coruscus and in vitro/in vivo evaluation of its bioactive properties. Fish Shellfish Immunol. 2013;34:1078–1084. doi: 10.1016/j.fsi.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Kumar NSS, Nazeer RA. Wound healing properties of collagen from the bone of two marine fishes. Int J Pept Res Ther. 2012;18:185–192. doi: 10.1007/s10989-012-9291-2. [DOI] [Google Scholar]
- Kumar S, Sugihara F, Suzuki K, Inoue N, Sriraam VT. A double-blind, placebo-controlled, randomised, clinical study on the effectiveness of collagen peptide on osteoarthritis. J Sci Food Agric. 2015;95:702. doi: 10.1002/jsfa.6752. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia J, Lehocky M, Humpolicek P, Saha P. HaCaT keratinocytes response on antimicrobial atelocollagen substrates: extent of cytotoxicity, cell viability and proliferation. J Funct Biomater. 2014;5:43–57. doi: 10.3390/jfb5020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- Matmaroh K, Benjakul S, Prodpran T, Encarnacion AB, Kishimura H. Characteristics of acid soluble collagen and pepsin soluble collagen from scale of spotted golden goatfish (Parupeneus heptacanthus) Food Chem. 2011;129:1179–1186. doi: 10.1016/j.foodchem.2011.05.099. [DOI] [PubMed] [Google Scholar]
- Minh Thuy le T, Okazaki E, Osako K. Isolation and characterization of acid-soluble collagen from the scales of marine fishes from Japan and Vietnam. Food Chem. 2014;149:264–270. doi: 10.1016/j.foodchem.2013.10.094. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Oftedal OT, Eisert R, Barrell GK. Comparison of analytical and predictive methods for water, protein, fat, sugar, and gross energy in marine mammal milk. J Dairy Sci. 2014;97:4713–4732. doi: 10.3168/jds.2014-7895. [DOI] [PubMed] [Google Scholar]
- Palanikumar L, Kumaraguru AK, Ramakritinan CM. Biochemical and genotoxic response of naphthalene to fingerlings of milkfish Chanos chanos. Ecotoxicology. 2013;22:1111–1122. doi: 10.1007/s10646-013-1098-1. [DOI] [PubMed] [Google Scholar]
- Ramshaw JA, Shah NK, Brodsky B. Gly-X-Y tripeptide frequencies in collagen: a context for host–guest triple-helical peptides. J Struct Biol. 1998;122:86–91. doi: 10.1006/jsbi.1998.3977. [DOI] [PubMed] [Google Scholar]
- Ravishankara MN, Shrivastava N, Padh H, Rajani M. Evaluation of antioxidant properties of root bark of Hemidesmus indicus R. Br. (Anantmul) Phytomedicine Int J Phytother Phytopharmacol. 2002;9:153–160. doi: 10.1078/0944-7113-00104. [DOI] [PubMed] [Google Scholar]
- Ray G, Husain SA. Oxidants, antioxidants and carcinogenesis. Indian J Exp Biol. 2002;40:1213–1232. [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cationdecolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Saluja S, Fabi S. A holistic approach to antiaging as an adjunct to antiaging procedures: a review of the literature. Dermatol Surg. 2017;43:475–484. doi: 10.1097/DSS.0000000000001027. [DOI] [PubMed] [Google Scholar]
- Shanmugam V, Ramasamy P, Subhapradha N, Sudharsan S, Seedevi P, Moovendhan M, Krishnamoorthy J, Shanmugam A, Srinivasan A. Extraction, structural and physical characterization of type I collagen from the outer skin of Sepiella inermis (Orbigny, 1848) Afr J Biotechnol. 2012;11:14326–14337. doi: 10.5897/AJB12.444. [DOI] [Google Scholar]
- Silva TH, Moreira-Silva J, Marques AL, Domingues A, Bayon Y, Reis RL. Marine origin collagens and its potential applications. Mar Drugs. 2014;12:5881–5901. doi: 10.3390/md12125881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontakke SB, Jung JH, Piao Z, Chung HJ. Orally available collagen tripeptide: enzymatic stability, intestinal permeability, and absorption of Gly-Pro-Hyp and Pro-Hyp. J Agric Food Chem. 2016;64:7127–7133. doi: 10.1021/acs.jafc.6b02955. [DOI] [PubMed] [Google Scholar]
- Venkatesan J, Anil S, Kim SK, Shim MS. Marine fish proteins and peptides for cosmeceuticals: a review. Mar Drugs. 2017;15:143. doi: 10.3390/md15050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, An X, Yang F, Xin Z, Zhao L, Hu Q. Isolation and characterisation of collagens from the skin, scale and bone of deep-sea redfish (Sebastes mentella) Food Chem. 2008;108:616–623. doi: 10.1016/j.foodchem.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Wu R, Wu C, Liu D, Yang X, Huang J, Zhang J, Liao B, He H, Li H. Overview of antioxidant peptides derived from marine resources: the sources, characteristic, purification, and evaluation methods. Appl Biochem Biotechnol. 2015;176:1815–1833. doi: 10.1007/s12010-015-1689-9. [DOI] [PubMed] [Google Scholar]
- Wu R, Chen L, Liu D, Huang J, Zhang J, Xiao X, Lei M, Chen Y, He H. Preparation of antioxidant peptides from salmon byproducts with bacterial extracellular proteases. Mar Drugs. 2017;15:4. doi: 10.3390/md15010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zhou X, Yi J, Tong J, Wu H, Fan L. Preparation and biological activity of quaternized carboxymethyl chitosan conjugated with collagen peptide. Int J Biol Macromol. 2014;70:300–305. doi: 10.1016/j.ijbiomac.2014.06.045. [DOI] [PubMed] [Google Scholar]