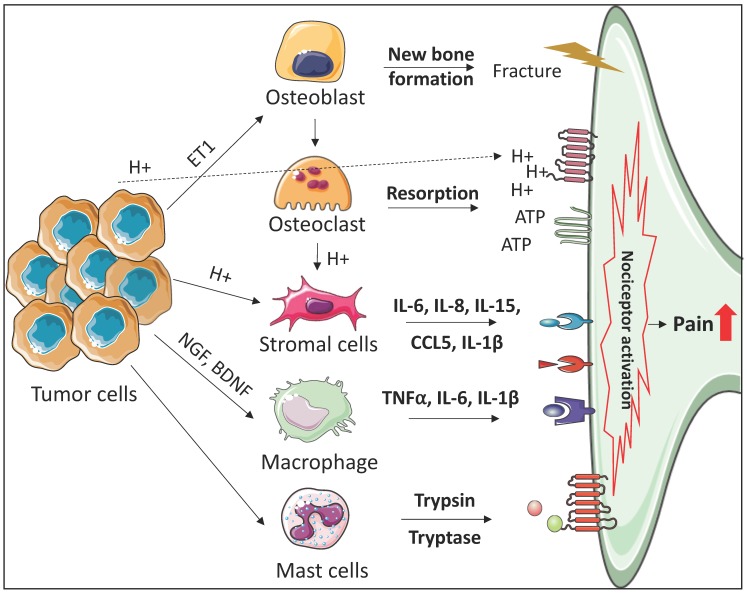
Figure 1.
Mechanisms of bone microenvironment involvement in cancer-induced bone pain. Bone-disseminated tumor cells release factors (e.g., ET1) to stimulate the proliferation of osteoblasts (e.g., endothelin A/B receptors), resulting in new bone formation, which is structurally weak and prone to fracture. Active osteoblasts release RANKL to promote osteoclast activity, resulting in increased bone resorption which also weakens bone. During bone resorption, nociceptors become sensitized and activated through osteoclast mediated acidification and ATP accumulation, which activates the acid sensing TRPV1 and ASICS receptors, or the ATP-gated P2X receptors expressed on sensory neurons, respectively. Tumor cell derived H+ directly induces nociception via activation of the acid sensing receptors expressed on the sensory neurons. Stromal cells (e.g., fibroblasts and mesenchymal stem cells) also express acid sensing receptors, and acidification of the bone marrow space stimulates release of stromal cell derived pro-inflammatory cytokines (IL-6, IL-8, IL-15, CCL5, IL-1β) and nociceptive mediators (NGF and BDNF). Tumor cells also express NGF and BDNF, which activate macrophages to release pro-inflammatory cytokines (TNF-α, IL-6, IL-1β) and inflammatory regulators (NGF and Prostaglandins) which directly induce pain via binding to their receptors on sensory neurons. Finally, tumor cells interact with peri-neural and tumor-infiltrated mast cells, releasing mast cell derived proteases (trypsin and tryptase) which activate sensory neurons by binding to PAR-2 receptor, resulting in pain and upregulation of pain-related neuropeptides (CGRP and SP).