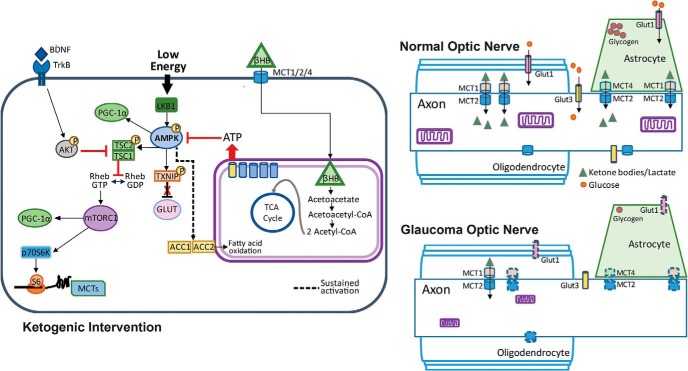
Figure 9.
Glucose and monocarboxylate transporter complement in optic nerve, and intracellular pathways regulating the response to low energy and ketogenic diet. Right, Top, Normal optic nerve showing mitochondria within the axon, and glycogen storage in the astrocyte contacting the axonal node of Ranvier. The glucose transporters GLUT1 (on astrocytes and oligodendrocytes) and GLUT3 (on axons) are distributed across their respective cells. The monocarboxylate transporters (MCT2 on axons, MCT1 on oligodendrocytes, and MCT1 and MCT4 on astrocytes) display kinetics that favor the movement of ketone bodies and lactate from the glia to the neurons. Right, Bottom, Glaucomatous optic nerve shows a reduction in mitochondria size (Coughlin et al., 2015), as well as decreased GLUT1 and MCT1, MCT2, and MCT4 transporters (denoted by dashed outlines). Left, Low energy levels lead to the activation of AMPK and the subsequent upregulation of PGC-1α and activation of TXNIP, thereby relieving the constitutive degradation of GLUT1. Activated (phosphorylated) AMPK also phosphorylates TCS2, thereby blocking the conversion of Rheb-GDP to Rheb-GTP and preventing the activation of the mTORC1. Sustained activation of AMPK upregulates ACC1 and ACC2, promoting fatty acid oxidation. Fatty acid oxidation and utilization of βHB provides ATP, thereby blocking AMPK activation. When not active, AMPK no longer blocks mTORC1 activity, allowing the activation of p70S6K, the phosphorylation of S6, and the subsequent translation of proteins such as MCT2. TXNIP, Thioredoxin-interacting protein; TCS2, tuberous sclerosis complex 2; ACC, acetyl-CoA carboxylase.