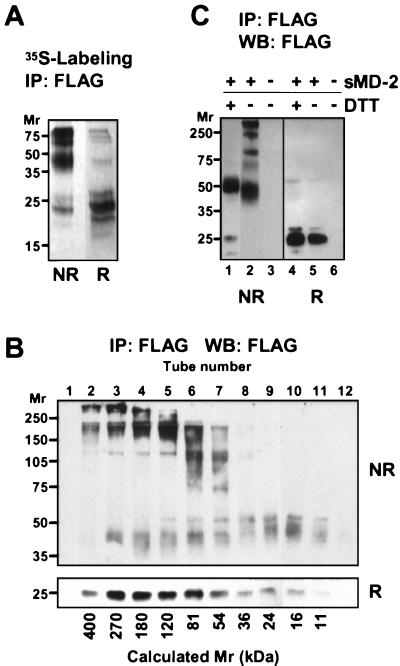
Figure 4.
sMD-2 forms large, disulfide-bonded aggregates. (A) 293T cells transfected with MD-2 were labeled overnight with 35S-Met. MD-2 was immunoprecipitated from the supernatant with an anti-FLAG mAb and subjected to SDS/PAGE under nonreducing (NR) or reducing (R) conditions. Shown is an autoradiogram after 2 days of exposure. (B) Concentrated supernatant (200 μl) from 293MD-2 cells was passed over a Superdex 200 HR 10/30 gel filtration column, and 1-ml fractions were collected immediately after the void volume. Fractions were immunoprecipitated, separated by SDS/10% PAGE under nonreducing (NR) and reducing (R) conditions, and blotted with HRP-anti-FLAG. No FLAG immunoreactivity was detected elsewhere in either membrane. Calculated molecular weights based on the elution of standard proteins are indicated at the bottom of the panel. (C) Mild reduction of sMD-2 yields disulfide-linked dimers. Immunoprecipitated sMD-2 was reduced (lanes 1 and 4) or not (lanes 2 and 5) with 5 mM DTT under nondenaturing conditions and resolved by SDS/10% PAGE under nonreducing (NR) and reducing (R) conditions. The gel was transferred and blotted with the anti-FLAG mAb as in 1B.