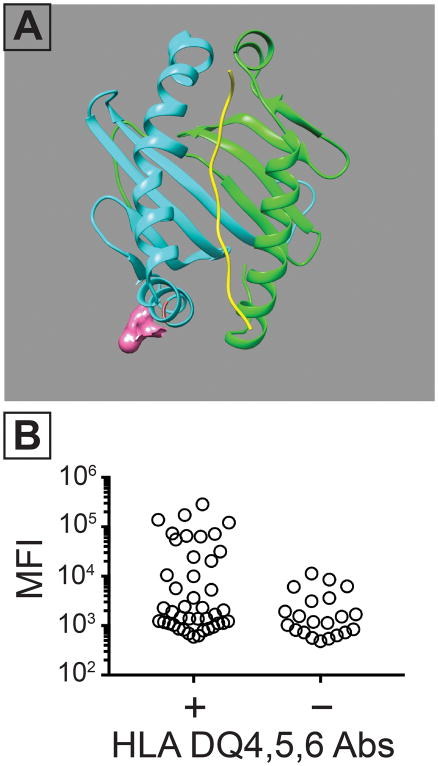
Figure 5. Amino acid 55 contributes to a class II SLA epitope which cross-reacts with human IgG.
Following the results in Figure 4, sequence alignments of HLA-DQ4,5,6, SLA- DQα1*0101/DQβ1*0601 were performed. These suggested that arginine at position 55 contributed to the cross-reactive epitope in DQβ and in the HLA beta chains in HLA-DQ4,5,6. (A) Structural prediction highlighting the putative location of the 55R residue in the SLA- DQα1*0101/DQβ1*0601 molecule. This residue was mutated to a proline (R55P) which is found in class II HLA molecules other than -DQ4,5,6 HLA molecules. (B) Human IgG binding to a cell line expressing CD74, SLA-DQα1*0101, and SLA-DQβ1*0601. All tested sera contained some class II HLA-binding IgG. The samples were split into two groups based on the presence (n = 42) or absence (n = 21) of HLA-DQ4,5,6 reactivity. The presence of antibodies against HLA-DQ4,5,6 increased binding to SLA-DQα1*0101, and DQβ1*0601(Mann-Whitney test, p= 0.030).