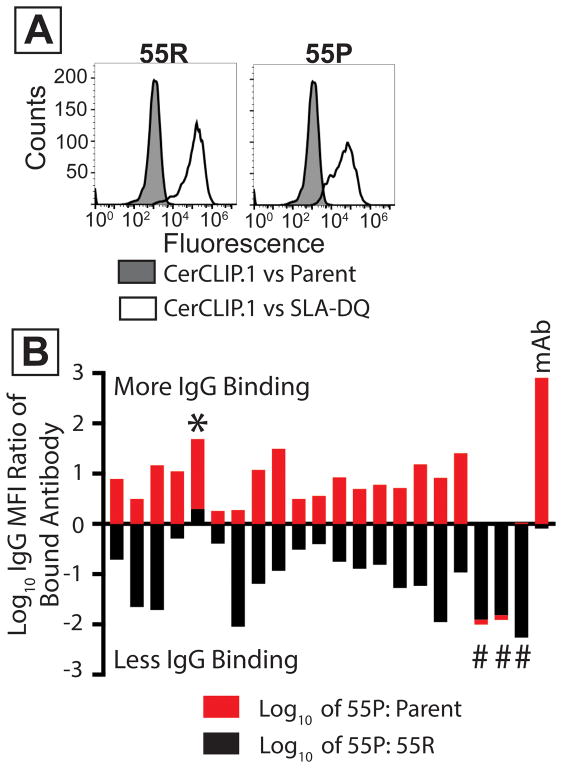
Figure 6. Mutation of arginine 55 in SLA-DQ beta reduces class II SLA-based xenoantigenicty.
Cell lines expressed CD74, SLA-DQα1*0101, and mutant or unmodified SLA-DQβ1*0601. Mutant molecules contained a β chain having proline at position 55 (55P). Wild type class II contained arginine at position (55R). (A) White histograms show binding of the CerCLIP.1 antibody to cells expressing either 55R or 55P. Gray histograms show CerCLIP.1 binding to CD74/SLA-deficient HEK 293T cells. (B) 21 human sera were incubated with cells expressing either the 55R or 55P variants of SLA-DQ. These sera were selected from prior experiments suggesting they reacted with SLA-DQα1*0101/SLA-DQβ1*0601. Data from each sample is represented by one red and one black bar (stacked on top of each other). MFI ratios of fluorescent anti-human IgG were calculated to compare binding to 55P versus 55R molecules. The log10 values of these ratios were plotted for each serum (black bars). The same analysis compared IgG binding to the 55P cells versus SLA-negative parent cells (red bars). The 55P mutation partially reduced IgG binding to the SLA-DQ protein in 17 samples. Three samples had no detectable antibody staining of 55P mutants (#). One sample showed greater binding to the 55P mutant than to 55R (*). The mAB sample indicates the binding of a monoclonal antibody specific for SLA-DQ. This antibody recognized SLA-DQ 55P variants (red bar) almost identically to 55R variants (black bar, Log10 of 55P/55R = −0.09).