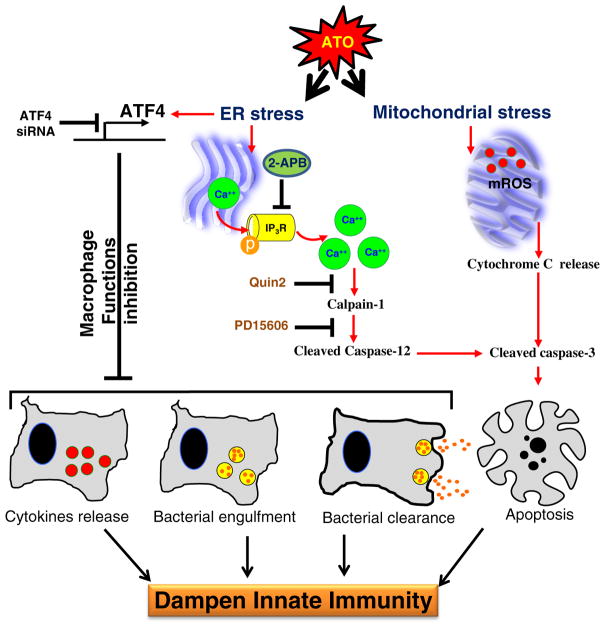
Fig. 7.
Flow diagram depicting the mechanism by which ATO treatment blocks macrophage functions and induces apoptosis in ATF4-dependent manner. ATO activates ATF4, a UPR signaling transcription factor, in murine macrophages, which dysregulates multiple macrophage functions including cytokines release, bacterial engulfment and clearance of engulfed bacteria. In ATO-treated macrophages, sustained activation of ATF4 leads to apoptosis via multiple pathways. Ca++-dependent calpain-1/caspase-12-mediated apoptosis and mitochondrial-dependent apoptosis via release of cytochrome-c from mitochondria to cytoplasm have been recorded. Overall, these effects may lead to dampening of macrophage-dependent innate immune responses. The role of ATF4 in ATO-mediated macrophage dysregulation was ascertained by the genetic approaches where knocking down of ATF4 afforded significantly protection against ATO-mediated impairment of macrophage functions. Role of calcium homeostasis in this toxicity could be confirmed by the treatment of these macrophages with Ca++ channel blocker or Ca++ chelators which attenuated ATO-induced calpain-1/caspase-12-mediated apoptosis and perhaps other functions related to ATF4 activation.