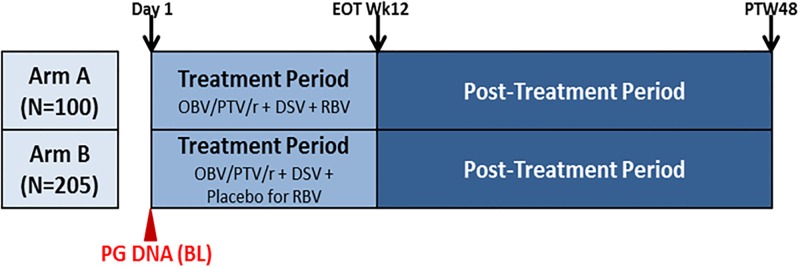
Fig 1. Study schematic for M14-002 (PEARL-IV).
During treatment period subjects received 12 weeks of therapy of OBV/PTV/r (150/100/25 mg QD) + DSV (250 mg BID) + RBV (or placebo for RBV). In the Post-Treatment Period, all subjects administered at least one dose of study drugs were followed for 48 weeks to monitor for safety, HCV RNA, the emergence and/or persistence of resistant viral variants and assessment of patient-reported outcomes.