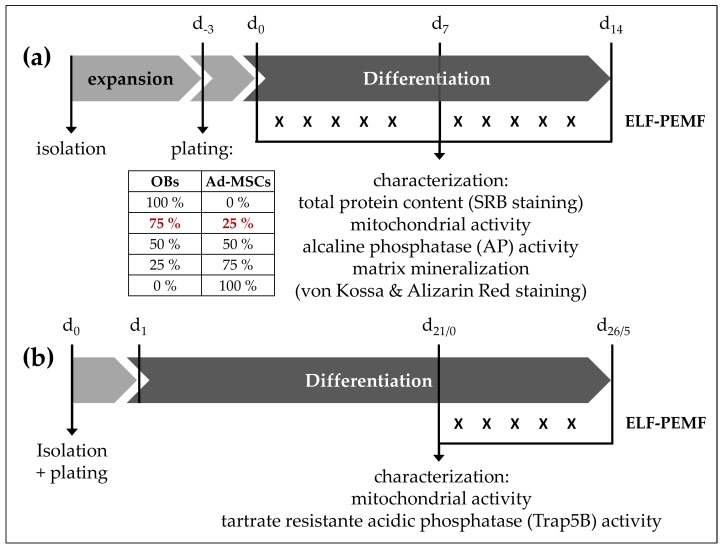
Figure 5.
Experimental setup. (a) OBs and Ad-MSCs: Cells were plated either as mono- or co-culture with different ratios. After complete adherence (3 days), growth medium was replaced by osteogenic differentiation medium. Medium was replaced every 3–4 days. During the entire differentiation period, cells were exposed daily to ELF-PEMF (16 or 26 Hz) for 7 min (for 5 consecutive days). On day 0, 7, and 14, total protein content (SRB staining), mitochondrial activity (resazurin conversion), AP activity, and matrix mineralization (alizarin red and von Kossa staining) were assessed. The red numbers 75% (OB) and 25% (Ad-MSCs) indicate the co-culture ratio with the best results. (b) Generated OCs: Mononuclear cells were isolated from peripheral blood via density gradient centrifugation. After 24 h, non-adherent cells were washed off the plate, and cells were exposed to osteoclastic (de-)differentiation medium, as indicated in Materials and Methods. Medium was replaced every 3–4 days over the course of 21 days. After 21 days (indicated as d21/0), the generated OCs were exposed daily to ELF-PEMF (16 or 26 Hz) for 7 min each day for the following 5 days. Before and after ELF-PEMF exposure, mitochondrial activity and Trap5B activity were measured. “X” indicates the daily 7 min exposure of the cells or cell cultures to ELF-PEMF.