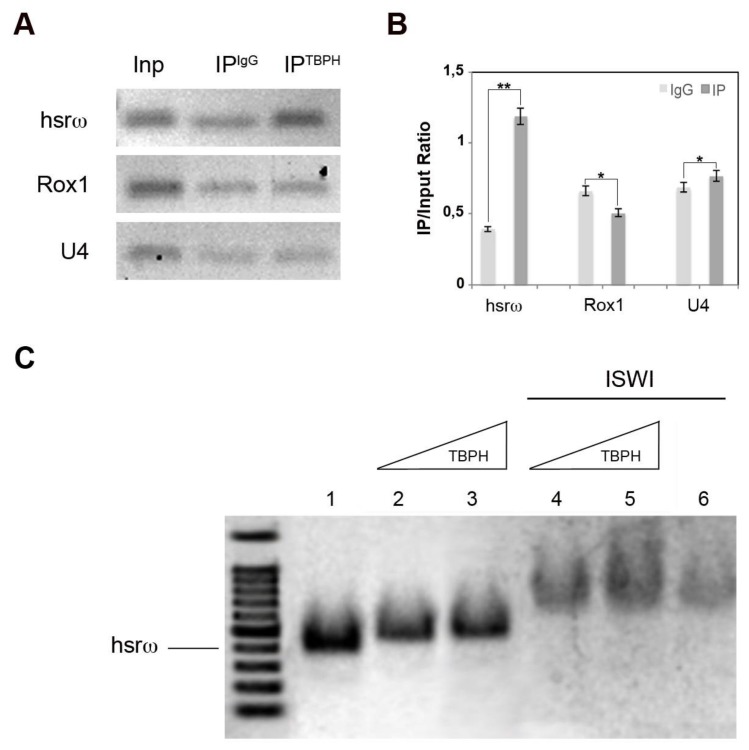
Figure 3.
TBPH physically and functionally interacts with the hsrω ncRNA in vitro. (A) Cross-linking RNA-immuno-precipitation (CLIP-RIP) assay was carried out with affinity purified anti-TBPH antibody on fixed larval nuclear extracts from the brain. The immunoprecipitate was analyzed by real-time polymerase chain reaction (RT-PCR) using primers for the 280 bp tandem repeat unit of the nuclear hsrω ncRNA; primers amplifying the U4 and Rox1 ncRNAs were used as specificity controls. Inp = input, IPTBPH = immunoprecipitated material from anti-TBPH CLIP, IPIgG = immunoprecipitated material from IgG CLIP; (B) The intensity of PCR signals was quantified using ImageJ. The experiment was performed considering five biological replicates. The error bars show the standard deviation. Unpaired Student’s t-test was performed to assay statistical significance; * 0.01 ≤ p-value ≤ 0.05; ** p-value < 0.01; (C) TBPH hnRNP binds α32P labeled hsrω-n arcRNA in electrophoresis shift mobility assay (EMSA). ISWI modulates the TBPH-hsrω interaction, as shown by changes in the gel shift. Binding reactions were performed at different stoichiometric ratios for hsr-ω/TBPH: 1:2 (lanes 2 and 4), 1:50 (lanes 3 and 5) and constant ratio for hsr-ω/ISWI: 1:20 (lanes 4, 5, and 6). Lane 1 shows the α32P hsrω-n probe only.